Kala-azar (Visceral Leishmaniasis)
A Comprehensive Guide for Nursing Students
Community Health Nursing Perspectives
Table of Contents
Introduction to Kala-azar
Kala-azar, also known as Visceral Leishmaniasis (VL), is a potentially fatal protozoan parasitic disease caused by the Leishmania donovani complex. The term “Kala-azar” originates from Hindi, meaning “black fever” – referring to the characteristic darkening of skin that occurs in patients from the Indian subcontinent. As one of the most severe forms of leishmaniasis, Kala-azar primarily attacks the internal organs, particularly the liver, spleen, and bone marrow.
From a community health nursing perspective, Kala-azar represents a significant public health challenge, especially in endemic regions. It is classified as a Neglected Tropical Disease (NTD) by the World Health Organization (WHO), affecting some of the poorest populations worldwide. Without proper treatment, the fatality rate for Kala-azar can exceed 95%.
Key Facts about Kala-azar:
- Second-largest parasitic killer globally after malaria
- Affects approximately 50,000-90,000 people annually
- Endemic in more than 70 countries across Asia, Africa, South America, and Southern Europe
- Transmitted by the bite of infected female phlebotomine sandflies
- Disproportionately affects vulnerable populations with limited access to healthcare
Epidemiology of Kala-azar
Understanding the epidemiology of Kala-azar is essential for nursing interventions at the community level. The disease exhibits distinct geographical patterns and seasonal variations that influence prevention strategies and control measures.
Global Distribution
Approximately 90% of Kala-azar cases occur in just six countries: Brazil, Ethiopia, India, Kenya, Somalia, and Sudan. The disease manifests in three epidemiological patterns:
Anthroponotic Transmission
Human-to-human transmission via sandflies. Predominant in the Indian subcontinent and East Africa.
Zoonotic Transmission
Animal reservoir (primarily dogs) to humans via sandflies. Common in the Mediterranean basin, Middle East, and Brazil.
Sporadic Transmission
Occasional cases with no clear pattern. Seen in non-endemic regions with travelers or immunocompromised individuals.
Epidemiological Trends
The incidence of Kala-azar has shown significant fluctuations over the decades:
- Historical epidemics in India killed hundreds of thousands in the early 20th century
- Resurgence in the 1970s after initial control with DDT spraying for malaria control
- Contemporary co-infection patterns with HIV that enhance disease severity and transmission
- Increasing cases in previously non-endemic areas due to climate change affecting vector distribution
| Endemic Region | Primary Vector | Causative Species | Key Epidemiological Features |
|---|---|---|---|
| Indian Subcontinent | Phlebotomus argentipes | Leishmania donovani | Anthroponotic transmission; post-kala-azar dermal leishmaniasis (PKDL); high population density |
| East Africa | P. orientalis, P. martini | L. donovani | Associated with Acacia-Balanites forests; nomadic populations; sporadic outbreaks |
| Mediterranean Basin | P. perniciosus, P. ariasi | L. infantum | Zoonotic; dogs as main reservoir; children and immunocompromised adults at risk |
| Brazil/Latin America | Lutzomyia longipalpis | L. infantum | Zoonotic; urbanization of cases; canine reservoir; increasing rural-to-urban spread |
Factors Influencing Epidemiology
Environmental Factors:
- Temperature (25-28°C optimal for sandfly breeding)
- Humidity (60-70% relative humidity)
- Rainfall patterns affecting vector breeding sites
- Altitude (typically below 700m)
- Vegetation (specific associations with Acacia and Balanites trees)
Human Factors:
- Population movement and displacement
- Urbanization and deforestation
- Housing conditions (mud walls, dampness)
- Proximity to animal reservoirs
- Socioeconomic status and access to healthcare
- Immunosuppression (especially HIV co-infection)
Epidemiological Surveillance Tips for Nurses:
- Maintain high index of suspicion in endemic areas for patients with prolonged fever
- Document case clustering by household and geography
- Record seasonal patterns to anticipate surge periods
- Integrate Kala-azar surveillance with existing health information systems
- Report all confirmed cases to national disease surveillance systems
Parasite Lifecycle and Transmission
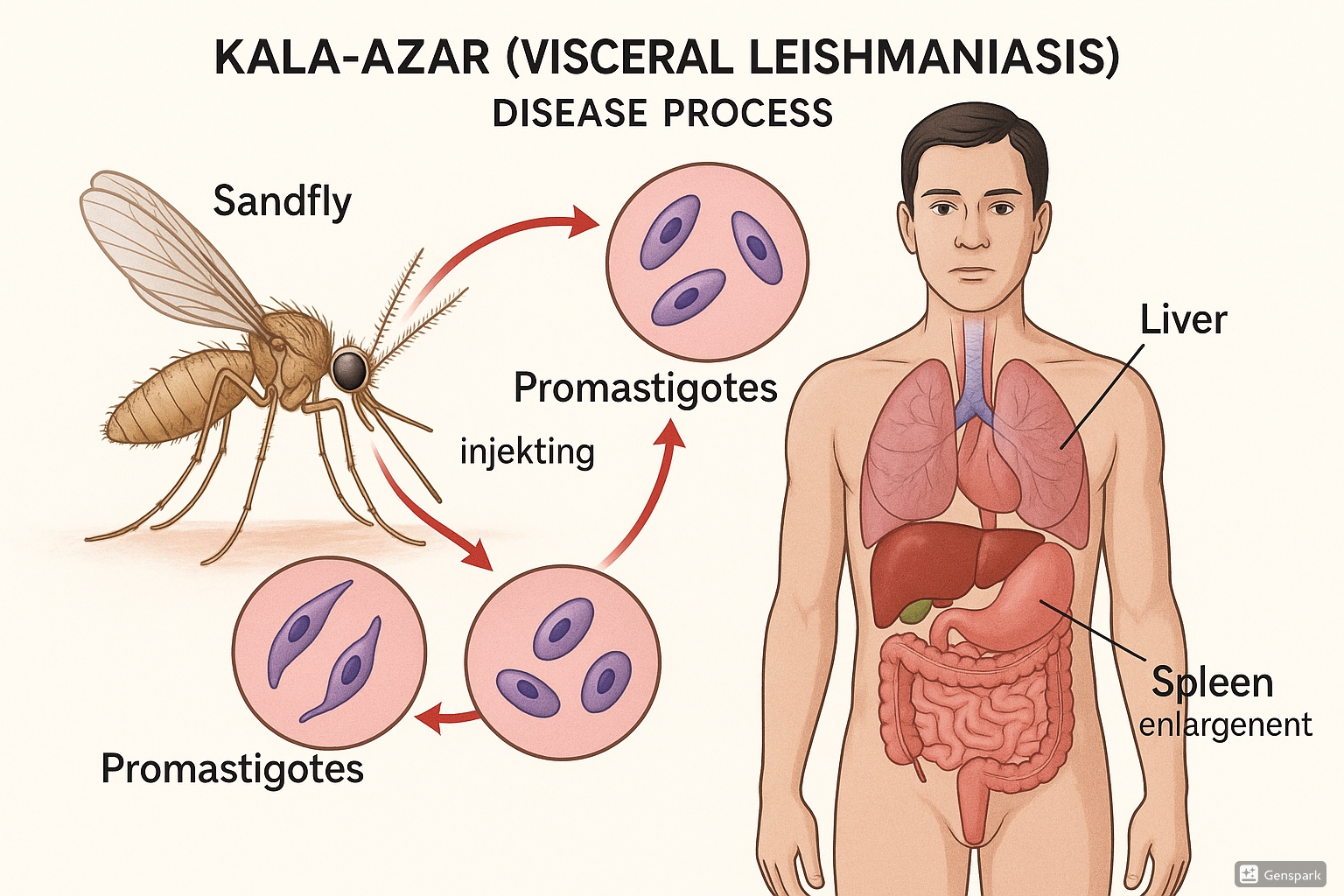
Figure 1: Life cycle of Leishmania parasite causing Kala-azar, showing transmission via sandfly and affected organs
Understanding the lifecycle of the Leishmania parasite is fundamental to comprehending Kala-azar transmission dynamics and implementing effective control strategies. The Kala-azar infection cycle involves two distinct hosts: the sandfly vector and the mammalian host (humans or animals).
The Parasite Lifecycle
In the Sandfly:
- Female sandfly ingests infected macrophages containing amastigotes during blood meal from infected host
- Amastigotes transform into promastigotes in the midgut of the sandfly
- Promastigotes multiply and migrate to the proboscis (mouthparts)
- Metacyclic promastigotes develop – the infective form ready for transmission
In the Human Host:
- Promastigotes injected into skin during sandfly blood meal
- Parasites are phagocytized by macrophages
- Within macrophages, promastigotes transform into amastigotes
- Amastigotes multiply by binary fission, rupturing cells and infecting other macrophages
- Infected macrophages disseminate to reticuloendothelial system (liver, spleen, bone marrow)
Transmission Dynamics
Kala-azar transmission is influenced by several key factors:
Vector Characteristics
- Female phlebotomine sandflies (2-3 mm in length)
- Most active during dusk and dawn
- Flight range limited to 300-500 meters
- Breed in organic matter, cracks in walls, and animal burrows
- Require temperatures between 15-38°C for survival
Reservoir Hosts
- Anthroponotic: Humans (especially PKDL cases) serve as reservoirs
- Zoonotic: Dogs are primary reservoirs
- Secondary animal reservoirs may include rodents, foxes, and jackals
- Asymptomatic human carriers may contribute to transmission
Transmission Seasons
- Typically highest during warm, humid months
- Indian subcontinent: April to October
- East Africa: Variable, often following rainfall patterns
- Mediterranean: May to September
- Incubation period: 2-6 months (range: 10 days to years)
Mnemonic: “VECTOR” for Kala-azar Transmission
V – Vulnerable populations at highest risk
E – Environmental factors influence transmission
C – Cyclic transmission requires two hosts
T – Tiny sandfly vector is the transmitter
O – Organism targets reticuloendothelial system
R – Reservoir hosts maintain the parasite cycle
Pathophysiology of Kala-azar
The pathophysiology of Kala-azar involves complex interactions between the Leishmania parasite and the host immune system. Understanding these mechanisms is crucial for nurses to comprehend the clinical manifestations and management approaches.
Invasion and Establishment
When introduced into human skin through a sandfly bite, Leishmania promastigotes are:
- Rapidly phagocytized by resident dermal macrophages and neutrophils
- Transformed into amastigotes within phagolysosomes of macrophages
- Able to survive within macrophages by inhibiting lysosomal enzymes and producing antioxidants
- Capable of modulating macrophage signaling to prevent activation and parasite killing
Systemic Dissemination
The hallmark of Kala-azar is the systemic dissemination of parasites to organs of the reticuloendothelial system:
Spleen
- Massive splenomegaly (can be 3-10 times normal size)
- Disruption of splenic architecture
- Hyperplasia of reticuloendothelial cells
- Parasitized macrophages throughout red and white pulp
- Increased risk of splenic rupture
Liver
- Moderate to marked hepatomegaly
- Kupffer cell hyperplasia and parasitization
- Inflammatory infiltrates in portal areas
- Variable hepatocyte injury
- Fibrosis in chronic cases
Bone Marrow
- Hyperplasia of macrophages
- Erythroid hypoplasia
- Displacement of normal hematopoietic cells
- Parasitized macrophages throughout
- Leads to pancytopenia
Immunopathogenesis
The immune response in Kala-azar is characterized by:
- Initial suppression of cell-mediated immunity (decreased Th1 response)
- Increased humoral immune response with hypergammaglobulinemia (ineffective against intracellular parasites)
- Polyclonal B-cell activation leading to production of non-specific antibodies
- Reduced interferon-gamma (IFN-γ) production by T cells
- Increased interleukin-10 (IL-10) contributing to disease progression
- Immune complex formation leading to glomerulonephritis and vasculitis
Systemic Complications
| System | Pathophysiological Changes | Clinical Implications |
|---|---|---|
| Hematological | Bone marrow infiltration, splenic sequestration, immune-mediated destruction | Pancytopenia, bleeding, susceptibility to secondary infections |
| Cardiovascular | Myocarditis, pericarditis, vasculitis | Heart failure, pericardial effusion, arrhythmias |
| Renal | Immune complex deposition, interstitial nephritis | Proteinuria, hematuria, acute kidney injury |
| Gastrointestinal | Intestinal parasitization, protein loss, malabsorption | Diarrhea, malnutrition, wasting |
| Integumentary | Melanocyte stimulation (Indian VL), post-kala-azar dermal leishmaniasis | Hyperpigmentation, skin lesions, potential reservoir for transmission |
Post-Kala-azar Dermal Leishmaniasis (PKDL)
A unique dermatological complication occurring in 5-10% of treated patients in India and up to 50% in Sudan:
- Develops months to years after apparent cure
- Characterized by macular, papular, or nodular skin lesions
- Results from sequestered parasites and immune reconstitution
- Serves as reservoir for continued transmission in anthroponotic cycle
- Requires specific treatment approach distinct from visceral disease
Clinical Manifestations of Kala-azar
Kala-azar typically presents with a gradual onset of symptoms that evolve over weeks to months. As community health nurses, recognizing these patterns is essential for early detection and intervention.
Classic Clinical Presentation
Mnemonic: “FEVER SPIKES” for Kala-azar Manifestations
F – Fever (irregular, prolonged)
E – Emaciation and weight loss
V – Visceral enlargement (hepatosplenomegaly)
E – Edema (peripheral, facial)
R – Respiratory complications
S – Skin hyperpigmentation
P – Pancytopenia
I – Infections (secondary bacterial)
K – Kidney involvement
E – Epistaxis and bleeding
S – Severe weakness
Stages of Clinical Progression
Early Stage (1-2 months)
- Intermittent fever patterns
- Fatigue and malaise
- Mild weight loss
- Initial splenomegaly (often detectable)
- Mild hepatomegaly
- Might be mistaken for malaria or typhoid
Established Disease (3-6 months)
- Persistent fever with evening spikes
- Significant weight loss
- Marked splenomegaly (palpable well below costal margin)
- Moderate to severe hepatomegaly
- Lymphadenopathy (common in African VL)
- Hyperpigmentation (common in Indian VL)
- Progressive anemia and weakness
Advanced Disease (>6 months)
- Cachexia and severe wasting
- Massive splenomegaly (can reach pelvis)
- Severe pancytopenia
- Bleeding manifestations (epistaxis, petechiae)
- Edema and ascites
- Secondary bacterial infections
- Jaundice and icterus
- Renal dysfunction
- High risk of mortality without treatment
Special Clinical Considerations
Kala-azar in Children
- Often more acute presentation than adults
- Pronounced growth retardation
- Rapid development of malnutrition
- More frequent diarrhea and respiratory complications
- Higher risk of concurrent infections
- May present with atypical features delaying diagnosis
Kala-azar in HIV Co-infection
- Atypical presentations common
- May involve unusual sites (GI tract, lungs, skin)
- Lower sensitivity of serological tests
- More rapid disease progression
- Higher rates of treatment failure
- Frequent relapses
- Higher mortality rates
Warning Signs Requiring Urgent Attention
- Severe bleeding (hematemesis, melena)
- Signs of bacterial sepsis
- Jaundice with altered mental status
- Severe anemia (Hb < 5 g/dL)
- Significant respiratory distress
- Severe dehydration or malnutrition
- Rapid enlargement of spleen (risk of rupture)
- Neurological manifestations
- Acute kidney injury
- Pregnancy with Kala-azar
Risk Factors for Kala-azar
Understanding risk factors for Kala-azar is essential for community health nurses to identify vulnerable populations and implement targeted interventions. These risk factors span individual, household, community, and environmental dimensions.
Individual Risk Factors
Demographic Factors
- Age: varies by region (children in Mediterranean basin, all ages in Indian subcontinent)
- Gender: often male predominance due to occupational exposure
- Genetic susceptibility: certain HLA types associated with higher risk
- Blood group: some studies indicate blood group A may have higher susceptibility
Host Conditions
- Immunocompromised states (particularly HIV infection)
- Malnutrition and micronutrient deficiencies
- Previous splenectomy
- Immunosuppressive medications
- Concurrent chronic diseases
- Pregnancy (altered immune status)
Household and Community Factors
Housing Characteristics
- Mud or thatched houses
- Cracked walls providing sandfly breeding sites
- Damp floors and poor ventilation
- Absence of bed nets or window screens
- Proximity to vegetation and water bodies
- Indoor storage of firewood or organic material
Occupational Risk
- Agricultural workers
- Forest workers and loggers
- Construction workers at new settlement sites
- Military personnel in endemic areas
- Nomadic populations
- Outdoor sleeping habits
- Migration for seasonal work
Community Factors
- Poverty and poor sanitation
- Limited access to healthcare
- Presence of untreated cases (human reservoirs)
- High density of domestic dogs (in zoonotic areas)
- Poor waste management
- Limited health literacy
- Population displacement and conflict
Environmental and Ecological Factors
| Environmental Factor | Impact on Kala-azar Risk | Regional Considerations |
|---|---|---|
| Climate Change | Expanding vector habitats to new areas; altering seasonal transmission patterns | Increasing cases in previously non-endemic higher altitude areas in East Africa and South America |
| Deforestation and Land Use | Creating new ecological niches; bringing humans into contact with sylvatic cycles | Particularly important in Brazil and other parts of Latin America |
| Urbanization | Introducing disease into peri-urban slums with poor living conditions | Growing concern in Brazil, Sudan, and parts of India |
| Altitude | Traditionally limited to lower altitudes (<700m); changing with climate shifts | Highland areas in Ethiopia now reporting increasing cases |
| Rainfall Patterns | Affecting vector breeding and activity; drought may concentrate hosts around water sources | Seasonal transmission peaks vary by region based on rainfall patterns |
Nursing Assessment Tips for Risk Stratification
When conducting community assessments in endemic areas, consider:
- Creating risk maps of communities based on environmental and housing characteristics
- Screening households with previous Kala-azar cases more frequently
- Prioritizing surveillance in areas with known environmental risk factors
- Identifying high-risk occupational groups for targeted education
- Assessing migration patterns that may introduce cases from endemic areas
- Monitoring HIV prevalence in regions with Kala-azar co-endemicity
- Evaluating household protective practices (bed nets, insecticide use, etc.)
Prevention and Control Measures for Kala-azar
Effective prevention and control of Kala-azar require a comprehensive approach targeting the parasite, vector, reservoirs, and human hosts. Community health nurses play a pivotal role in implementing and monitoring these strategies.
Vector Control Strategies
Indoor Residual Spraying (IRS)
- Spraying insecticides on interior walls of houses
- Targets resting sandflies
- Commonly used insecticides: pyrethroids, DDT (where permitted)
- Requires 6-monthly application in most settings
- Most effective in anthroponotic transmission areas
- Challenges: insecticide resistance, proper application techniques
Environmental Management
- Filling cracks and crevices in house walls
- Proper waste management to reduce breeding sites
- Clearing vegetation around houses (>5 meters)
- Maintaining dry surroundings to reduce humidity
- Avoiding accumulation of organic matter near living areas
- Plastering walls with lime-based materials
Personal Protection Methods
- Use of long-lasting insecticidal nets (LLINs)
- Wearing long-sleeved clothing in endemic areas
- Application of insect repellents (DEET-based)
- Installation of fine-mesh screens on windows and doors
- Avoiding outdoor activities during peak sandfly biting times (dusk to dawn)
- Sleeping in well-protected areas
Chemical Control Methods
- Insecticide-treated curtains and wall linings
- Insecticide-treated dog collars (in zoonotic areas)
- Space spraying during outbreaks
- Larvicidal applications in identified breeding sites
- Fumigation of vulnerable spaces
- Use of insecticidal baits
Reservoir Control
Human Reservoir Management
- Early case detection and treatment
- Active surveillance in endemic communities
- Treatment of PKDL cases to reduce transmission
- Contact tracing of confirmed cases
- Management of asymptomatic carriers (controversial)
- Special attention to HIV co-infected individuals
Animal Reservoir Control (Zoonotic VL)
- Screening and treatment of infected dogs
- Culling of infected dogs (in some countries)
- Canine vaccination (experimental)
- Insecticide-treated dog collars
- Regular application of topical insecticides on dogs
- Control of stray dog populations
- Wildlife management in sylvatic cycles
Integrated Control Approaches
The Five Pillars of Kala-azar Control
- Early Diagnosis and Complete Treatment – Reducing disease burden and preventing complications
- Vector Control – Reducing human-vector contact
- Effective Disease Surveillance – Monitoring trends and detecting outbreaks
- Social Mobilization and Behavior Change – Engaging communities in prevention
- Operational Research – Improving strategies and addressing implementation gaps
Community Health Nursing Interventions
| Intervention Level | Key Activities | Nursing Responsibilities |
|---|---|---|
| Primary Prevention | Health education, vector control, environmental management |
|
| Secondary Prevention | Early case detection, screening, prompt treatment |
|
| Tertiary Prevention | Rehabilitation, prevention of complications, follow-up care |
|
Health Education and Behavior Change
Key Messages
- Disease transmission and risk factors
- Early warning signs and symptoms
- Importance of early healthcare seeking
- Need for complete treatment adherence
- Personal protection methods
- Housing improvement techniques
- Community participation in control efforts
Delivery Methods
- Community meetings and workshops
- School-based education programs
- House-to-house visits
- Mass media campaigns (radio, TV)
- Information, education, communication (IEC) materials
- Peer education and community champions
- Mobile health (mHealth) initiatives
Target Audiences
- General population in endemic areas
- High-risk occupational groups
- Local healthcare providers
- Community leaders and influencers
- School children as change agents
- Migrant populations
- Political and administrative stakeholders
Mnemonic: “PREVENT” for Kala-azar Control
P – Protect with bed nets and screens
R – Reduce sandfly breeding sites
E – Early diagnosis and treatment
V – Vector control through IRS
E – Educate communities about risks
N – Nutritional support for vulnerable groups
T – Track and monitor cases systematically
Screening and Diagnosis of Kala-azar
Timely and accurate diagnosis of Kala-azar is critical for effective management and control. Community health nurses play an important role in screening efforts, initial assessment, and facilitating diagnostic procedures.
Screening Approaches
Community-based Screening
- Active case finding in endemic communities
- House-to-house fever surveys
- Screening of household contacts of confirmed cases
- Camp-based approach during outbreaks
- Integration with other disease screening programs
- Use of community health workers for preliminary screening
Target Population Screening
- Individuals with prolonged fever (>2 weeks)
- Patients with splenomegaly of unknown cause
- Unexplained weight loss and anemia
- Children with growth faltering in endemic areas
- HIV-positive individuals in co-endemic regions
- Migrants from endemic regions with compatible symptoms
- Individuals with post-kala-azar dermal leishmaniasis (PKDL)
Clinical Assessment
Clinical Case Definition for Suspecting Kala-azar
A person with:
- Fever for more than 2 weeks
- Splenomegaly
- AND either:
- Weight loss, weakness, or
- Anemia (pallor)
- AND living in or having traveled to an endemic area
Nursing Assessment Components
- History taking: Duration of fever, pattern, associated symptoms, travel history, occupational exposure, previous treatment
- Physical examination: Focus on spleen size, liver enlargement, lymphadenopathy, nutritional status, skin changes, signs of bleeding
- Vital signs: Fever pattern documentation, weight monitoring, signs of dehydration
- Risk assessment: Evaluation of household conditions, vector exposure, proximity to other cases
Initial Screening Tests
- rK39 rapid diagnostic test: Field-applicable immunochromatographic test detecting antibodies
- Direct agglutination test (DAT): Serological test with high sensitivity/specificity but more complex than RDTs
- Complete blood count: Assessment for pancytopenia (anemia, leukopenia, thrombocytopenia)
- Basic metabolic panel: Evaluation of renal function, liver enzymes, albumin levels
- Urinalysis: Checking for proteinuria, hematuria
Diagnostic Methods
| Diagnostic Test | Sensitivity/Specificity | Advantages | Limitations | Setting |
|---|---|---|---|---|
| Parasitological Methods (Gold Standard) | Bone marrow: 60-85% Spleen: 93-99% Lymph node: 52-58% |
Definitive diagnosis Species identification possible |
Invasive Requires skilled personnel Risk of complications (splenic aspiration) |
Tertiary hospitals Reference laboratories |
| rK39 Rapid Diagnostic Test | Sensitivity: 92-100% Specificity: 80-100% (varies by region) |
Field-applicable Quick results (20 min) Minimal training needed Affordable |
Lower sensitivity in East Africa Remains positive after cure Cross-reactivity with other diseases Less sensitive in HIV co-infection |
Primary health centers Field settings Community screening |
| Direct Agglutination Test (DAT) | Sensitivity: 94-98% Specificity: 95-98% |
High reliability Can be used with blood spots Less affected by geographical variation |
Requires laboratory setup Overnight incubation Reader variability Cold chain requirements |
District hospitals Reference laboratories |
| PCR-based Methods | Sensitivity: 92-99% Specificity: 100% |
High sensitivity/specificity Species identification Can detect low parasite loads Quantification possible |
Expensive Requires sophisticated laboratory Technical expertise needed Limited field application |
Reference laboratories Research settings |
| LAMP (Loop-mediated Isothermal Amplification) | Sensitivity: 80-98% Specificity: 94-100% |
Simpler than PCR Field-adaptable Rapid results Less equipment needed |
Still requires basic lab capacity Limited commercial availability Quality control issues |
District hospitals Some primary health centers |
Diagnostic Algorithm in Resource-Limited Settings
- Initial screening: Clinical case definition + rK39 RDT
- If positive: Treat as Kala-azar (in highly endemic areas with typical presentation)
- If negative but strong clinical suspicion: Perform DAT (if available) or refer to higher center
- In referral centers: Parasitological confirmation through bone marrow or splenic aspiration
- In complicated/atypical cases: Additional tests including PCR, culture, and histopathology
- In HIV co-infection: Lower threshold for parasitological diagnosis due to reduced sensitivity of serological tests
Differential Diagnosis
Infectious Causes
- Malaria (especially chronic forms)
- Typhoid fever
- Tuberculosis (disseminated)
- Brucellosis
- Histoplasmosis
- Schistosomiasis
- HIV/AIDS
- Infectious mononucleosis
Hematological Causes
- Leukemia
- Lymphoma
- Myelodysplastic syndromes
- Myelofibrosis
- Hemolytic anemia
- Tropical splenomegaly syndrome
Other Conditions
- Autoimmune disorders (SLE)
- Cirrhosis with portal hypertension
- Sarcoidosis
- Amyloidosis
- Gaucher’s disease
- Congestive heart failure
Nursing Role in Diagnostic Procedures
- Preparation and education: Explain procedures to patients and families, obtain informed consent
- Sample collection: Assist with collection of blood samples, prepare patients for specialized procedures
- Procedure assistance: Support clinicians during bone marrow or splenic aspirations
- Post-procedure care: Monitor for complications after invasive procedures (especially splenic aspiration)
- Documentation: Record test results, maintain diagnostic registers, ensure follow-up
- Specimen handling: Proper collection, labeling, storage, and transport of specimens
- Point-of-care testing: Perform and interpret rK39 RDTs with proper quality control
Primary Management of Kala-azar
The primary management of Kala-azar requires a comprehensive approach including pharmacological treatment, supportive care, and management of complications. Community health nurses are integral to ensuring treatment adherence, monitoring, and educating patients and families.
Pharmacological Treatment
| Drug | Dosage and Duration | Advantages | Limitations/Side Effects | Nursing Considerations |
|---|---|---|---|---|
| Liposomal Amphotericin B (First-line in most regions) |
3-5 mg/kg/day IV for 3-5 days OR Single dose of 10 mg/kg |
High efficacy (95-98%) Short treatment course Low toxicity WHO recommended |
High cost IV administration Cold chain requirement Infusion reactions Hypokalemia |
Monitor for infusion reactions Assess renal function Check electrolytes Maintain aseptic technique Monitor for fever/chills |
| Miltefosine | Adults: 50 mg BID for 28 days Children: 2.5 mg/kg/day for 28 days |
Oral administration Outpatient treatment Good efficacy in most regions |
Teratogenic (contraindicated in pregnancy) GI side effects Hepatotoxicity Long treatment course Compliance issues |
Ensure contraception in women of childbearing age Monitor for GI symptoms Regular liver function tests Emphasize compliance for full course |
| Paromomycin | 11 mg/kg/day IM for 21 days | Low cost Good efficacy in most regions Short half-life |
IM administration (painful) Ototoxicity Nephrotoxicity Variable efficacy by region |
Rotate injection sites Monitor renal function Assess hearing Evaluate for vestibular symptoms Observe for local reactions |
| Sodium Stibogluconate (SSG) (Pentavalent antimonials) |
20 mg/kg/day IV/IM for 30 days | Long history of use Relatively low cost Effective in sensitive areas |
Significant resistance in many regions Cardiotoxicity, hepatotoxicity, pancreatitis Painful injections Long treatment course |
ECG monitoring Regular cardiac assessment Monitor liver enzymes and amylase Assess for arthralgias Careful administration technique |
| Combination Therapy (e.g., SSG + Paromomycin) |
SSG 20 mg/kg + Paromomycin 15 mg/kg daily for 17 days | Reduced treatment duration Lower resistance development Cost-effective High efficacy |
Combined side effect profiles Multiple daily injections Monitoring complexity |
Monitor for overlapping toxicities Organize dual medication schedules Comprehensive side effect monitoring Patient education on multiple drugs |
Special Treatment Considerations
HIV Co-infection:
- Liposomal Amphotericin B is preferred (total dose: 40 mg/kg)
- Higher relapse rates; secondary prophylaxis may be needed
- ART should be initiated/continued
- More intensive monitoring required
- Extended treatment courses often necessary
Pregnancy:
- Liposomal Amphotericin B is safest option
- Miltefosine is contraindicated
- Antimonials generally avoided (risk of abortion)
- Close obstetric monitoring required
- Treatment should not be delayed
Supportive Care
Nutritional Support
- Protein-rich diet for tissue repair
- High-calorie intake to combat wasting
- Micronutrient supplementation (especially iron, folate, vitamin B12)
- Therapeutic feeding for severe malnutrition
- Frequent, small meals if appetite poor
- Regular weight monitoring
- Consideration of enteral feeding in severe cases
Management of Anemia
- Blood transfusion for severe anemia (Hb <7 g/dL with symptoms or <5 g/dL regardless)
- Iron supplementation after parasite clearance
- Regular hemoglobin monitoring
- Assessment for bleeding sources
- Oxygen therapy when indicated
- Activity modification based on anemia severity
Managing Secondary Infections
- Regular screening for bacterial infections
- Empiric antibiotics for suspected infections
- Pneumonia prevention and management
- Tuberculosis screening in endemic areas
- Skin care to prevent infections
- Oral hygiene to prevent mucositis
- Urinary tract infection prevention
Nursing Management Plan
Key Nursing Interventions for Kala-azar Patients
Assessment and Monitoring:
- Regular vital signs monitoring (especially temperature patterns)
- Daily weight measurement
- Spleen size measurement and documentation
- Fluid intake and output recording
- Assessment for bleeding manifestations
- Monitoring for drug side effects
- Regular laboratory parameter review
Direct Care Interventions:
- Medication administration according to protocol
- IV line care and management
- Position changes to prevent pressure ulcers
- Assisted ambulation as tolerated
- Tepid sponging for fever
- Skin care, especially in edematous areas
- Oral care for mucositis and dehydration prevention
Management of Complications
| Complication | Management Approach | Nursing Responsibilities |
|---|---|---|
| Bleeding (thrombocytopenia, DIC) | Platelet transfusion if <10,000 or active bleeding Fresh frozen plasma for coagulopathy Vitamin K supplementation Avoid IM injections if possible |
Monitor for bleeding sites Apply pressure to bleeding areas Minimize trauma during procedures Educate on bleeding precautions Monitor transfusion reactions |
| Bacterial Sepsis | Blood cultures before antibiotics Broad-spectrum antibiotics Source identification and control Fluid resuscitation as needed Vasopressors for septic shock |
Early recognition of sepsis signs Accurate fluid administration Strict infection control Frequent vital signs monitoring Implementation of sepsis protocols |
| Severe Malnutrition | Staged nutritional rehabilitation Treatment of specific deficiencies Monitoring for refeeding syndrome Multivitamin supplementation Nutritional counseling |
Gradual refeeding protocol Accurate weight monitoring Calorie counting and documentation Assessment for edema Electrolyte monitoring |
| Renal Dysfunction | Dose adjustment of nephrotoxic drugs Fluid management Electrolyte correction Renal replacement therapy if severe |
Strict fluid balance monitoring Assessment of urine output Monitoring of renal function tests Recognition of uremic symptoms Drug dose timing and adjustment |
| PKDL (Post-kala-azar Dermal Leishmaniasis) | Specific treatment protocols Longer treatment courses Miltefosine or Amphotericin B Regular follow-up of lesions |
Skin assessment and documentation Patient education on infectivity Treatment adherence support Photography of lesions to monitor progress Follow-up scheduling |
Mnemonic: “TREATED” for Kala-azar Management
T – Therapeutic drugs administered correctly
R – Rehydration and electrolyte balance
E – Education of patient and family
A – Anemia and bleeding management
T – Treat secondary infections promptly
E – Ensure nutritional rehabilitation
D – Document and monitor response
Referral Criteria for Kala-azar
Appropriate and timely referral is essential in the management of Kala-azar, especially for cases presenting with complications or in vulnerable populations. Community health nurses must be familiar with referral criteria to ensure optimal patient outcomes.
Primary to Secondary/Tertiary Care Referral
Urgent/Emergency Referral Criteria
- Severe bleeding manifestations (hematemesis, melena, excessive epistaxis)
- Signs of septic shock (hypotension, altered mental status)
- Severe anemia (Hb <5 g/dL) with cardiorespiratory compromise
- Suspected splenic rupture (acute abdominal pain, hypotension)
- Altered consciousness or neurological manifestations
- Severe dehydration unresponsive to oral rehydration
- Significant electrolyte abnormalities
- Jaundice with signs of hepatic encephalopathy
- Acute kidney injury (oliguria, anuria, rising creatinine)
- Severe drug reactions (anaphylaxis, Stevens-Johnson syndrome)
Non-Urgent Referral Criteria
- Diagnostic uncertainty requiring specialized testing
- Treatment failure (no clinical improvement after 2 weeks of therapy)
- Relapse cases requiring alternative treatment
- Comorbidities complicating management:
- HIV co-infection
- Tuberculosis
- Chronic liver or kidney disease
- Diabetes with poor control
- Pregnancy with Kala-azar
- Children under 2 years of age
- Elderly patients with multiple comorbidities
- PKDL requiring specialized management
- Drug toxicity requiring modification of treatment
Referral Protocol
Components of Effective Kala-azar Referral
- Pre-referral Stabilization:
- Secure IV access if not already present
- Initial fluid resuscitation for dehydration or shock
- First dose of antibiotics for suspected sepsis
- Control active bleeding sites
- Position appropriately for transport (e.g., left lateral for massive splenomegaly)
- Documentation and Communication:
- Structured referral note with clinical findings
- Test results and diagnostic information
- Treatment already administered
- Reason for referral clearly stated
- Contact information of referring facility
- Advanced communication with receiving facility when possible
- Transport Considerations:
- Appropriate transport mode based on urgency
- Accompaniment by trained healthcare worker for unstable patients
- Necessary equipment for monitoring during transport
- Medications that may be needed en route
- Clear directions to receiving facility for family if separate transport
Levels of Care Matrix
| Level of Care | Capabilities | Patient Presentations Appropriate for Level | When to Refer to Next Level |
|---|---|---|---|
| Community/Village Health Worker | Suspect cases Basic first aid Referral to primary care |
Initial screening of fever cases Follow-up of treated cases Community education |
All suspected Kala-azar cases Any fever >2 weeks with splenomegaly |
| Primary Health Center | rK39 testing Basic laboratory tests Uncomplicated case management Oral drugs administration |
Uncomplicated, RDT-positive cases Stable patients suitable for miltefosine Follow-up of referred cases |
Negative RDT but strong clinical suspicion Children <2 years Pregnant women Any complications Treatment failure |
| District/Secondary Hospital | DAT testing Bone marrow aspiration Amphotericin B administration Blood transfusion Management of moderate complications |
Complicated but stable cases RDT-negative cases requiring confirmation Cases requiring IV therapy Pediatric cases Mild to moderate anemia requiring transfusion |
Cases requiring intensive care Severe complications Diagnostic dilemmas Multiple treatment failures HIV co-infection Severe drug reactions |
| Tertiary/Specialty Center | PCR diagnosis Splenic aspiration ICU facilities Specialist consultations Dialysis capabilities Management of severe complications |
Critically ill patients Multiorgan involvement HIV co-infection Treatment failures Research protocols Unusual presentations |
Rarely needed; may refer to specialized research centers for experimental treatments in refractory cases |
Nursing Responsibilities in Referral Process
Before Referral
- Recognize early warning signs requiring referral
- Complete assessment documentation
- Stabilize patient appropriately
- Explain need for referral to patient/family
- Organize transport logistics
- Prepare referral documentation
- Contact receiving facility when possible
- Ensure essential medications accompany patient
During Referral
- Accompany unstable patients when required
- Monitor vital signs during transport
- Provide necessary interventions en route
- Maintain IV access and fluid therapy
- Administer medications as needed
- Document condition changes during transport
- Communicate with receiving facility about ETA
- Support family members during transfer
After Referral
- Complete referral register documentation
- Follow up on patient outcomes
- Receive feedback from higher facility
- Plan for return/follow-up care
- Update community health records
- Incorporate lessons learned for future cases
- Schedule follow-up visits after discharge
- Coordinate continuity of care
Best Practices for Effective Referral Systems
- Establish clear referral pathways with contact information for each level of care
- Develop standardized referral forms specific to Kala-azar
- Implement two-way communication systems between referring and receiving facilities
- Conduct regular training on referral criteria for all healthcare workers
- Create transportation arrangements or ambulance protocols for urgent cases
- Establish feedback mechanisms for referred cases
- Maintain a referral register for monitoring and evaluation
- Integrate Kala-azar referrals with existing health system referral structures
Follow-up Care for Kala-azar
Effective follow-up care is essential for monitoring treatment response, detecting complications or relapse, and preventing long-term sequelae. Community health nurses play a critical role in ensuring systematic follow-up and continuity of care.
Post-Treatment Follow-up Schedule
Recommended Follow-up Timeline
| Follow-up Timing | Clinical Assessment | Laboratory Tests | Key Interventions |
|---|---|---|---|
| 2 weeks post-treatment | Assessment of fever resolution Spleen and liver size Weight measurement Nutritional status Drug side effects evaluation |
Complete blood count Liver function tests Kidney function tests |
Nutritional counseling Side effect management Treatment of residual issues Address medication concerns |
| 1 month post-treatment | Reassessment of clinical improvement Spleen regression Weight gain General well-being Skin examination for PKDL |
Complete blood count Basic metabolic panel Test of cure (if indicated) |
Continued nutritional support Rehabilitation planning Reinforce preventive measures School/work reintegration guidance |
| 3 months post-treatment | Assessment for complete recovery Evaluation for relapse signs PKDL screening Physical activity tolerance Psychosocial adjustment |
Complete blood count Selected tests based on symptoms |
Health education reinforcement Family counseling Community reintegration Psychosocial support if needed |
| 6 months post-treatment | Final cure assessment Thorough PKDL screening Growth monitoring (children) Chronic sequelae evaluation |
Complete blood count Other tests if symptomatic |
Final nutritional assessment Long-term prevention education Community surveillance integration Discharge from active follow-up if cured |
| Additional follow-up (special cases) |
HIV co-infected: quarterly for 1 year PKDL cases: monthly until resolution Relapse cases: monthly for 6 months Complicated cases: individualized schedule |
Based on specific condition and complications | Secondary prophylaxis (HIV co-infection) Specialized treatment protocols Intensive monitoring Specialist consultation as needed |
Monitoring for Treatment Success
Clinical Indicators of Cure
- Complete resolution of fever
- Regression of splenomegaly (at least 50% of original size)
- Improvement in general condition and energy level
- Weight gain and improved appetite
- Resolution of anemia and other cytopenias
- Normalization of skin pigmentation (in Indian VL)
- Improved exercise tolerance
- Return to normal activities
Monitoring for Relapse
- Recurrence of fever after initial resolution
- Re-enlargement of spleen after regression
- Return of weakness and fatigue
- Weight loss after initial improvement
- Worsening or recurrent anemia
- New onset of bleeding manifestations
- Darkening of skin after initial lightening
- Recurrent infections
Post-Kala-azar Dermal Leishmaniasis (PKDL) Surveillance
Timing and Risk
- Indian subcontinent: Usually 6 months to several years after treatment
- East Africa (Sudan): Often within 6 months of treatment
- Occurs in 5-10% of treated cases in India
- Up to 50% of treated cases in Sudan
- Higher risk with incomplete treatment
- Important reservoir for continued transmission
Clinical Features
- Hypopigmented macules (early stage)
- Erythematous papules and nodules
- Facial distribution common (butterfly pattern)
- May involve oral mucosa in severe cases
- Progression from macular to nodular forms
- Usually non-painful and non-pruritic
- Can mimic leprosy, vitiligo, or cutaneous leishmaniasis
Management Approach
- Confirmation by skin slit smear or biopsy
- Extended treatment courses required
- Miltefosine for 12 weeks (preferred in India)
- Amphotericin B formulations as alternatives
- Monthly follow-up until resolution
- Documentation of lesions (photography helpful)
- Patient counseling on infection risk
Nursing Interventions During Follow-up
| Area of Focus | Nursing Interventions | Patient/Family Education |
|---|---|---|
| Physical Recovery |
|
|
| Psychological/Social Recovery |
|
|
| Prevention of Relapse/Reinfection |
|
|
Community-Based Follow-up Strategies
Strategies for Hard-to-Reach Areas
- Training community health workers for basic follow-up
- Mobile clinic services for periodic specialist review
- Integration with existing outreach programs
- Telemedicine consultations where infrastructure permits
- Simplified assessment tools for non-specialists
- Community-based monitoring systems
- Peer support groups for patients and families
- SMS/mobile phone reminders for follow-up dates
Follow-up Registers and Documentation
- Standardized follow-up registers with key indicators
- Patient-held records for continuity across facilities
- Follow-up cards with scheduled appointment dates
- Geographic mapping of cases for outreach planning
- Community-based surveillance integration
- Digital tracking systems where available
- Monitoring and evaluation frameworks
- Data analysis for identifying loss to follow-up patterns
Mnemonic: “FOLLOWS” for Kala-azar Follow-up Care
F – Frequent scheduled assessments
O – Observe for relapse or PKDL
L – Laboratory monitoring as indicated
L – Lifestyle modification guidance
O – Ongoing nutritional support
W – Watching for complications
S – Systematic documentation and reporting
Nursing Care Plan for Kala-azar
A comprehensive nursing care plan is essential for providing systematic care to patients with Kala-azar. This care plan addresses the common nursing diagnoses and outlines specific interventions and expected outcomes.
| Nursing Diagnosis | Assessment Data | Nursing Interventions | Expected Outcomes |
|---|---|---|---|
| Hyperthermia related to infectious process as evidenced by elevated body temperature and chills |
|
|
|
| Imbalanced Nutrition: Less than body requirements related to increased metabolic demands, anorexia, and parasitic infection |
|
|
|
| Risk for Bleeding related to thrombocytopenia and coagulopathy secondary to disease process |
|
|
|
| Risk for Infection (secondary) related to immunosuppression, neutropenia, and invasive procedures |
|
|
