Table of Contents
- Introduction to Universal Immunization Programme
- Historical Background and Evolution
- Objectives and Goals of UIP
- Vaccine-Preventable Diseases under UIP
- Immunization Schedule
- Cold Chain Management
- Vaccine Administration Guidelines
- Role of Community Health Nurses in UIP
- Monitoring and Evaluation
- Challenges and Strategies
- Global Best Practices
Introduction to Universal Immunization Programme
The Universal Immunization Programme (UIP) is one of the largest public health initiatives in the world, aimed at reducing infant and child mortality caused by vaccine-preventable diseases. Launched in India in 1985, the Universal Immunization Programme (UIP) has become a cornerstone of preventive healthcare services.
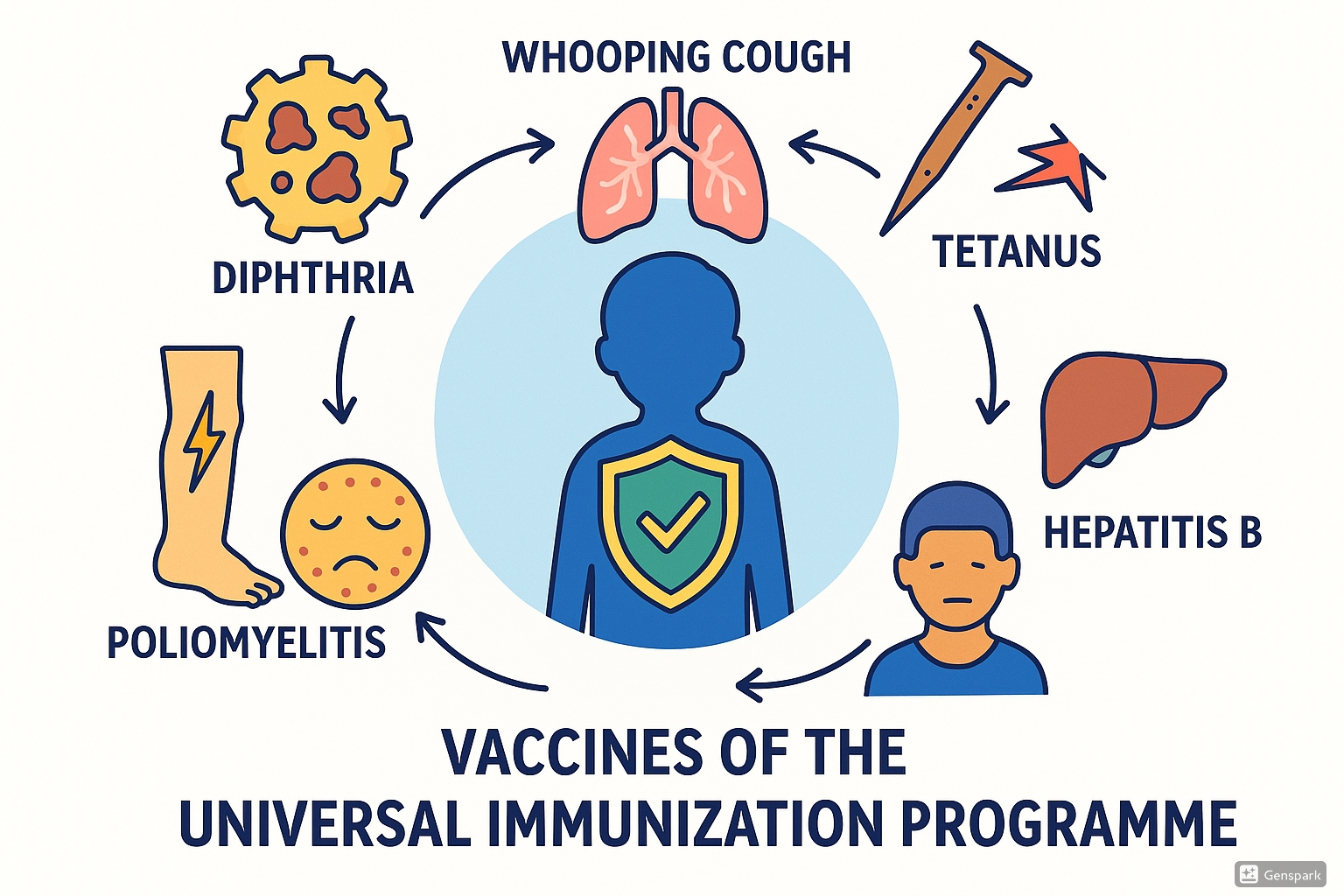
The Universal Immunization Programme initially targeted six vaccine-preventable diseases: Diphtheria, Pertussis (Whooping Cough), Tetanus, Poliomyelitis, Measles, and Tuberculosis. Hepatitis B was later added to the program, expanding its scope to protect against more life-threatening conditions.
Key Components of the Universal Immunization Programme:
- Provision of free vaccines against preventable diseases
- Establishment of cold chain infrastructure
- Training of healthcare workers for vaccine administration
- Community mobilization and awareness campaigns
- Surveillance and monitoring systems
The UIP functions through a vast network of healthcare facilities, from tertiary hospitals to sub-centers and outreach sessions, ensuring that immunization services reach every corner of the country. As community health nurses, understanding the Universal Immunization Programme is essential for effective implementation and advocacy within the community.
Historical Background and Evolution
Origins and Development
The journey of the Universal Immunization Programme began with the global efforts to eradicate smallpox. Following the success of the Smallpox Eradication Programme, the World Health Organization (WHO) launched the Expanded Programme on Immunization (EPI) in 1974 to tackle other vaccine-preventable diseases.
India adopted the EPI in 1978, focusing initially on urban areas. Recognizing the need for wider coverage, the programme was renamed the Universal Immunization Programme (UIP) in 1985 with the goal of extending immunization services to all districts in the country.
Timeline of UIP Evolution
| Year | Milestone |
|---|---|
| 1978 | Expanded Programme on Immunization (EPI) launched in India |
| 1985 | EPI renamed as Universal Immunization Programme (UIP) |
| 1990 | UIP became a part of Child Survival and Safe Motherhood (CSSM) Programme |
| 1997 | UIP integrated into Reproductive and Child Health (RCH) Programme |
| 2005 | Incorporated under National Rural Health Mission (NRHM) |
| 2010 | Introduction of Hepatitis B vaccine nationwide |
| 2017 | Launch of Mission Indradhanush to increase immunization coverage |
| 2018 | Intensified Mission Indradhanush to reach the unreached |
Expansion of Vaccine Coverage
Over the decades, the Universal Immunization Programme has expanded its vaccine portfolio. From the initial six vaccines, the program now provides protection against 12 vaccine-preventable diseases in many states, with newer vaccines like rotavirus, pneumococcal conjugate vaccine (PCV), and inactivated polio vaccine (IPV) being introduced in recent years.
Objectives and Goals of UIP
Primary Objectives
The Universal Immunization Programme (UIP) was established with clear objectives to improve the health outcomes of children and reduce mortality from preventable diseases.
Core Objectives of the Universal Immunization Programme:
- Reduce mortality and morbidity from vaccine-preventable diseases
- Achieve and maintain high immunization coverage
- Establish a reliable cold chain system for vaccine storage
- Develop a self-sufficient program for vaccine delivery
- Integrate immunization with other health services
Specific Goals
The UIP has set specific targets to measure its success and impact on public health:
| Goal | Target |
|---|---|
| Immunization Coverage | Achieve and maintain >90% full immunization coverage |
| Disease Reduction | Eliminate polio, neonatal tetanus, and reduce other vaccine-preventable diseases |
| Equity | Reduce immunization disparities between geographical areas and social groups |
| System Strengthening | Develop sustainable infrastructure and workforce capacity |
| Community Engagement | Increase awareness and community participation |
Alignment with Global Initiatives
The Universal Immunization Programme aligns with several global health initiatives and frameworks:
- Sustainable Development Goals (SDGs)
- Global Vaccine Action Plan (GVAP)
- Polio Eradication and Endgame Strategic Plan
- Global Strategy for Women’s, Children’s and Adolescents’ Health
Through these alignments, the UIP contributes to global efforts to improve health outcomes and reduce preventable deaths among children worldwide.
Vaccine-Preventable Diseases under UIP
The Universal Immunization Programme (UIP) targets six major vaccine-preventable diseases that pose significant health risks to infants and children. Understanding these diseases is crucial for community health nurses involved in implementing the UIP.
1. Diphtheria
Causative Agent
Corynebacterium diphtheriae, a gram-positive bacillus
Mode of Transmission
Respiratory droplets or direct contact with infected persons or carriers
Clinical Manifestations
- Sore throat and low-grade fever
- Characteristic grayish-white membrane over tonsils, pharynx, or larynx
- Neck swelling (“bull neck” appearance)
- Difficulty breathing and swallowing
- Potential cardiac and neurological complications
Prevention through UIP
Administered as part of DPT (Diphtheria, Pertussis, Tetanus) vaccine or Pentavalent vaccine
Vaccine Schedule
- Primary doses: 6, 10, and 14 weeks of age
- Booster doses: 16-24 months and 5-6 years
Nursing Considerations
- Monitor for local reactions at injection site
- Educate parents about possible mild fever post-vaccination
- Early recognition of symptoms for prompt treatment
Case Fatality Rate: 5-10% even with treatment, making prevention through Universal Immunization Programme crucial.
2. Whooping Cough (Pertussis)
Causative Agent
Bordetella pertussis, a gram-negative coccobacillus
Mode of Transmission
Airborne respiratory droplets; highly contagious
Clinical Manifestations
The disease progresses through three stages:
- Catarrhal Stage (1-2 weeks): Mild cough, runny nose, low-grade fever
- Paroxysmal Stage (2-4 weeks): Characteristic “whoop” sound after rapid coughing fits, vomiting after coughing
- Convalescent Stage (1-2 weeks): Gradual recovery with decreasing episodes
Prevention through UIP
Administered as part of DPT vaccine or Pentavalent vaccine
Vaccine Schedule
- Primary doses: 6, 10, and 14 weeks of age
- Booster dose: 16-24 months
Nursing Considerations
- Observe for post-vaccination fever and irritability
- Educate on importance of completing the full vaccine series
- Special attention needed for premature infants
- Recognize that immunity wanes over time
Mnemonic for Pertussis Stages: “CPC”
Catarrhal (mild symptoms) → Paroxysmal (whooping cough) → Convalescent (recovery)
3. Tetanus
Causative Agent
Clostridium tetani, an anaerobic gram-positive bacillus
Mode of Transmission
Entry of spores through wounds, cuts, or umbilical cord in newborns
Clinical Manifestations
- Muscle stiffness and spasms, typically beginning in jaw (lockjaw)
- Painful muscle contractions in neck, chest, back, and abdomen
- Difficulty swallowing
- Autonomic dysfunction (fever, sweating, tachycardia)
- In neonates: inability to suck, irritability, seizures
Prevention through UIP
Administered as part of DPT vaccine or Pentavalent vaccine; TT for pregnant women
Vaccine Schedule
- Infants: 6, 10, and 14 weeks (primary doses)
- Boosters: 16-24 months and 5-6 years
- Pregnant women: Two doses of TT/Td during first pregnancy
Nursing Considerations
- Proper wound care education
- Ensuring complete maternal immunization
- Promoting clean delivery practices
- Awareness about booster requirements
Special Focus: Neonatal Tetanus Elimination is a key goal of the Universal Immunization Programme, achieved by ensuring maternal immunization and clean delivery practices.
4. Poliomyelitis
Causative Agent
Poliovirus (types 1, 2, and 3), an enterovirus
Mode of Transmission
Fecal-oral route or, less commonly, oral-oral route
Clinical Manifestations
Polio presents with varied clinical patterns:
- Subclinical infection (90-95%): No symptoms
- Abortive poliomyelitis: Fever, sore throat, headache, vomiting
- Non-paralytic poliomyelitis: Meningeal irritation, back pain, neck stiffness
- Paralytic poliomyelitis: Acute flaccid paralysis, typically asymmetric, most often affecting legs
Prevention through UIP
Administered as Oral Polio Vaccine (OPV) and Inactivated Polio Vaccine (IPV)
Vaccine Schedule
- OPV: Birth dose (zero dose), then at 6, 10, and 14 weeks
- IPV: Two fractional doses at 6 and 14 weeks
- Additional OPV doses during Pulse Polio Immunization campaigns
Nursing Considerations
- Proper administration of oral drops
- Cold chain maintenance for vaccine potency
- Surveillance for Acute Flaccid Paralysis (AFP)
- Awareness of Vaccine-Associated Paralytic Poliomyelitis (VAPP)
Pulse Polio Strategy:
A key component of the Universal Immunization Programme involving nationwide synchronized campaigns to administer OPV to all children under five years, regardless of previous immunization status.
5. Measles
Causative Agent
Measles virus (Paramyxovirus family)
Mode of Transmission
Airborne respiratory droplets; highly contagious
Clinical Manifestations
Classic presentation follows a specific pattern:
- Prodromal phase (3-4 days): High fever, cough, coryza (runny nose), conjunctivitis (3Cs)
- Koplik spots: Small white spots with red base on buccal mucosa (pathognomonic)
- Rash phase: Maculopapular rash beginning at hairline, spreading downward to face, trunk, and extremities
- Complications: Pneumonia, encephalitis, diarrhea, blindness
Prevention through UIP
Administered as Measles vaccine or as Measles-Rubella (MR) vaccine
Vaccine Schedule
- First dose: 9-12 months of age
- Second dose: 16-24 months of age
Nursing Considerations
- Ensuring timely vaccination despite maternal antibodies
- Vitamin A supplementation with measles vaccination
- Monitoring for post-vaccination mild fever and rash
- Surveillance for measles outbreaks
Mnemonic for Measles Symptoms: “MEASLES”
Maculopapular rash, Encephalitis (complication), Airborne transmission, Sore eyes (conjunctivitis), Lethargy and fever, Ear infection (complication), Spots (Koplik’s)
6. Hepatitis B
Causative Agent
Hepatitis B virus (HBV), a DNA virus
Mode of Transmission
- Perinatal transmission from infected mother to child
- Contact with infected blood or body fluids
- Unsafe injections or medical procedures
- Sexual contact with infected individuals
Clinical Manifestations
Varies from asymptomatic to severe:
- Acute phase: Fatigue, nausea, jaundice, dark urine, abdominal pain
- Chronic infection: Often asymptomatic for years
- Long-term complications: Cirrhosis, liver failure, hepatocellular carcinoma
Prevention through UIP
Administered as Hepatitis B vaccine or as part of Pentavalent vaccine
Vaccine Schedule
- Birth dose (within 24 hours for institutional deliveries)
- 6, 10, and 14 weeks of age (as part of pentavalent vaccine)
Nursing Considerations
- Importance of birth dose for preventing vertical transmission
- Proper injection technique (intramuscular in anterolateral thigh)
- Educating on the long-term protection against liver diseases
- Screening of high-risk pregnant women
Critical Point: The birth dose of Hepatitis B vaccine within 24 hours can prevent 90-95% of mother-to-child transmission, highlighting the importance of institutional deliveries in the Universal Immunization Programme.
Immunization Schedule
The Universal Immunization Programme (UIP) follows a standardized immunization schedule to ensure optimal protection against vaccine-preventable diseases. Community health nurses must be thoroughly familiar with this schedule to provide timely and effective immunization services.
| Age | Vaccines | Route | Site | Dose |
|---|---|---|---|---|
| Birth (within 24 hours) | OPV-0 (Polio) Hepatitis B-Birth dose |
Oral IM |
– Anterolateral thigh |
2 drops 0.5 ml |
| 6 weeks | OPV-1 Pentavalent-1 (DPT + Hep B + Hib) fIPV-1 Rotavirus-1 PCV-1 |
Oral IM ID Oral IM |
– Anterolateral thigh Right upper arm – Left anterolateral thigh |
2 drops 0.5 ml 0.1 ml 5 drops 0.5 ml |
| 10 weeks | OPV-2 Pentavalent-2 Rotavirus-2 |
Oral IM Oral |
– Anterolateral thigh – |
2 drops 0.5 ml 5 drops |
| 14 weeks | OPV-3 Pentavalent-3 fIPV-2 Rotavirus-3 PCV-2 |
Oral IM ID Oral IM |
– Anterolateral thigh Left upper arm – Left anterolateral thigh |
2 drops 0.5 ml 0.1 ml 5 drops 0.5 ml |
| 9-12 months | MR-1 (Measles-Rubella) JE-1* Vitamin A (1st dose) |
SC SC Oral |
Right upper arm Left upper arm – |
0.5 ml 0.5 ml 1 ml (1 lakh IU) |
| 16-24 months | MR-2 OPV Booster DPT Booster-1 JE-2* PCV Booster |
SC Oral IM SC IM |
Right upper arm – Anterolateral thigh Left upper arm Left anterolateral thigh |
0.5 ml 2 drops 0.5 ml 0.5 ml 0.5 ml |
| 5-6 years | DPT Booster-2 | IM | Deltoid | 0.5 ml |
| 10 & 16 years | Td (Tetanus & diphtheria) | IM | Deltoid | 0.5 ml |
| Pregnant women | Td1/Td2/TdB** | IM | Upper arm | 0.5 ml |
* JE vaccine is given only in endemic districts
** Td1: First dose in early pregnancy, Td2: Second dose 4 weeks after Td1, TdB: Booster dose if previously vaccinated (within 3 years)
IM: Intramuscular, SC: Subcutaneous, ID: Intradermal
Key Points for Nurses Implementing the UIP Schedule:
- Maintain the recommended interval between doses for optimal immune response
- If the schedule is interrupted, resume without restarting the entire series
- Mild illnesses are not contraindications for vaccination
- Multiple vaccines can be administered during the same visit at different sites
- Document all vaccines in the immunization card and register
Mnemonic for Remembering UIP Schedule: “BORN TO PROTECT”
Birth: OPV-0, Hepatitis B birth dose
Onset at 6 weeks: First dose of Pentavalent, OPV, fIPV
Repeat at 10 & 14 weeks: Continuing doses
Nine months: MR first dose
Toward 16-24 months: Booster doses
Older child (5-6 years): DPT second booster
Pre-teen & teen: Td vaccine
Repeat for pregnant women: Td doses
Optimize coverage for all
Track carefully through documentation
Educate caregivers
Community health promotion
Timely administration is crucial
Cold Chain Management
Cold chain management is an essential component of the Universal Immunization Programme (UIP), ensuring that vaccines maintain their potency from manufacturer to administration. Community health nurses play a crucial role in maintaining this system at the last mile of delivery.
Cold Chain System Components
- Personnel: Cold chain handlers, vaccine logistic managers
- Equipment: Walk-in coolers, ice-lined refrigerators, deep freezers, cold boxes, vaccine carriers
- Transport: Refrigerated vans, insulated vehicles
- Monitoring devices: Temperature loggers, freeze indicators, VVM (Vaccine Vial Monitors)
- Procedures: Storage guidelines, contingency plans, preventive maintenance
Temperature Requirements
| Storage Level | Temperature Range | Storage Duration |
|---|---|---|
| Primary/National | -15°C to -25°C | 6-12 months |
| Regional/State | -15°C to -25°C | 3-4 months |
| District | +2°C to +8°C | 1-3 months |
| PHC/CHC | +2°C to +8°C | 1 month |
| Last mile (session) | +2°C to +8°C | 1 day |
Vaccine-Specific Storage Guidelines
| Vaccine | Storage Temperature | Freeze Sensitive | Heat Sensitive | Position in ILR |
|---|---|---|---|---|
| OPV | -15°C to -25°C (Freezer) +2°C to +8°C (ILR for use) |
No | High | Bottom shelf |
| Measles/MR | +2°C to +8°C | No | Medium | Bottom shelf |
| DPT | +2°C to +8°C | Yes | Low | Top shelf |
| Pentavalent | +2°C to +8°C | Yes | Low | Top shelf |
| TT/Td | +2°C to +8°C | Yes | Low | Top shelf |
| Hepatitis B | +2°C to +8°C | Yes | Medium | Top shelf |
| IPV/fIPV | +2°C to +8°C | Yes | Medium | Top shelf |
Vaccine Vial Monitor (VVM)
VVM is a time-temperature sensitive label attached to vaccine vials that indicates cumulative heat exposure through a gradual color change. It helps healthcare workers identify vaccines that may have been damaged by heat.
VVM Stages:
- Stage 1: Square lighter than circle – Use the vaccine
- Stage 2: Square still lighter than circle – Use the vaccine
- Stage 3: Square same color as circle – Do not use if other vials are available
- Stage 4: Square darker than circle – Never use, discard the vaccine
Shake Test for Freeze-Sensitive Vaccines
Used to determine if freeze-sensitive vaccines (DPT, TT, Hepatitis B, Pentavalent) have been damaged by freezing:
- Select a test vial suspected of freezing and a control vial (known to be not frozen)
- Shake both vials vigorously for 10-15 seconds
- Place both vials side by side on a flat surface against light
- Observe for 30 minutes, comparing sedimentation rate
- If test vial shows faster sedimentation with clear solution above, it has been frozen and should be discarded
Cold Chain Breach Protocol:
- Immediately report to the supervisor
- Quarantine affected vaccines but do not discard
- Record time and duration of the breach
- Check VVMs and perform shake test if applicable
- Document the incident and consult with immunization officer
- Follow state protocol for disposal of non-usable vaccines
Mnemonic for Cold Chain Maintenance: “COLD CHAIN”
Continuous monitoring of temperature
Organize vaccines properly in the refrigerator
Log temperature readings twice daily
Defrost regularly as per schedule
Check VVMs before use
Handle vaccines gently
Always maintain correct temperatures
Inventory management using FEFO (First Expiry, First Out)
Notify immediately if any breach occurs
Vaccine Administration Guidelines
Proper vaccine administration is crucial for ensuring the safety and efficacy of immunizations provided under the Universal Immunization Programme (UIP). Community health nurses must adhere to standardized techniques and protocols.
Pre-Administration Assessment
- Verify child’s identity and check immunization record
- Screen for contraindications and precautions
- Check vaccine expiry date and VVM status
- Counsel parents/caregivers about benefits and side effects
- Obtain verbal consent before administration
Contraindications
General Contraindications:
- Severe allergic reaction to previous dose
- Severe immunodeficiency (for live vaccines)
- Moderate to severe acute illness with or without fever
Not Contraindications:
- Mild illness with low-grade fever
- Current antibiotic therapy
- Recent exposure to infectious disease
- Prematurity (for age-appropriate vaccines)
- History of mild reactions to previous doses
Administration Techniques
| Route | Technique | Site | Examples |
|---|---|---|---|
| Intramuscular (IM) | 90° angle, Z-track technique recommended |
|
Pentavalent, DPT, TT/Td, Hepatitis B |
| Subcutaneous (SC) | 45° angle, pinch skin fold | Upper arm | Measles, MR, JE |
| Intradermal (ID) | 10-15° angle, bevel up | Upper arm | fractional-IPV (fIPV) |
| Oral | Directly into mouth | – | OPV, Rotavirus |
Safe Injection Practices
- Use auto-disable (AD) syringes for all immunizations
- Follow “no touch” technique
- Never recap needles after use
- Dispose of all used syringes in puncture-proof container
- One syringe – one vaccine – one child
- Maintain aseptic technique throughout
Post-Administration Care
- Monitor for immediate adverse events for 15-30 minutes
- Advise on common side effects and management
- Document vaccination in records (date, vaccine, batch number, site, VVM status)
- Schedule next visit for follow-up doses
- Update immunization card and registry
Adverse Events Following Immunization (AEFI)
An AEFI is any untoward medical occurrence that follows immunization but may not necessarily have a causal relationship with the vaccine. The Universal Immunization Programme has a structured AEFI surveillance system for monitoring and investigation.
| Category | Examples | Management |
|---|---|---|
| Minor | Fever, pain/swelling at injection site, mild rash | Symptomatic treatment, reassurance |
| Severe | High fever (>102°F), excessive crying, persistent vomiting | Prompt medical attention, documentation |
| Serious | Anaphylaxis, encephalopathy, seizures, intussusception (after rotavirus) | Emergency management, immediate reporting, investigation |
| Program-related | Injection site abscess, contaminated vaccines | Evaluate procedural gaps, improve practices |
| Coincidental | Events unrelated to vaccine but temporally associated | Investigation to determine causality |
Anaphylaxis Management – ABCDE Protocol:
Airway: Ensure airway is open and clear
Breathing: Administer oxygen if available
Circulation: Position patient flat with legs elevated
Drugs: Administer adrenaline 0.01 mg/kg (1:1000) IM
Evacuation: Transfer to nearest health facility immediately
Mnemonic for Safe Vaccine Administration: “RIGHT”
Right Vaccine: Verify the correct vaccine for the child’s age
Identify the child: Confirm identity and check immunization record
Get consent: Provide information and obtain verbal consent
Handling and technique: Use proper administration technique
Track and document: Record all details of immunization
Role of Community Health Nurses in UIP
Community health nurses are the cornerstone of the Universal Immunization Programme (UIP), serving as the primary point of contact for vaccine delivery and community engagement. Their multifaceted role encompasses various responsibilities crucial for the success of the program.
Clinical Roles
- Administer vaccines according to the UIP schedule
- Conduct pre-vaccination assessment
- Manage and report adverse events
- Maintain cold chain at the session site
- Implement infection control measures
Managerial Roles
- Plan and organize immunization sessions
- Maintain vaccine inventory and supplies
- Document and report immunization data
- Track defaulters and dropout cases
- Coordinate with ASHA workers, AWWs, and other stakeholders
Educational Roles
- Educate families about immunization benefits and schedule
- Address vaccine hesitancy and misconceptions
- Conduct health education sessions in communities
- Train frontline workers and volunteers
- Promote awareness of vaccine-preventable diseases
Strategies for Increasing Immunization Coverage
Community health nurses can implement several strategies to enhance Universal Immunization Programme coverage and effectiveness:
- Microplanning: Develop detailed plans to reach every child in the community, utilizing area maps and household surveys
- Tracking System: Implement a robust system to track eligible beneficiaries and follow up with dropouts
- Community Mobilization: Engage community leaders, religious figures, and influential persons to advocate for immunization
- Innovative Approaches: Mobile health teams, extended hours, using local events for outreach
- Integrated Service Delivery: Combine immunization with other maternal and child health services
- Communication Strategies: Utilize culturally appropriate messages and multiple channels (interpersonal, mass media, social media)
Addressing Vaccine Hesitancy
Vaccine hesitancy remains a significant challenge for the Universal Immunization Programme. Community health nurses need effective strategies to address concerns and misconceptions:
Effective Communication Techniques:
- Listen actively to concerns without judgment
- Provide accurate, evidence-based information
- Use simple language avoiding medical jargon
- Acknowledge side effects while emphasizing benefits
- Share personal experiences with vaccines
- Use visual aids to explain concepts
Common Concerns and Responses
| Concern | Effective Response |
|---|---|
| “Too many vaccines overwhelm the immune system” | “Infants encounter far more immune challenges daily than vaccines provide. The immune system can handle multiple vaccines safely.” |
| “Vaccines cause autism” | “Multiple large studies have found no link between vaccines and autism. This misconception came from a study that was later retracted.” |
| “Natural immunity is better” | “Natural immunity comes with disease risks including complications and death. Vaccines provide immunity without these dangers.” |
| “Vaccines contain harmful ingredients” | “Vaccine ingredients are in tiny amounts, tested for safety, and serve important functions. The body naturally processes these substances.” |
Documentation and Record-Keeping
Community health nurses are responsible for maintaining accurate immunization records, which are essential for the Universal Immunization Programme’s success:
- Mother and Child Protection (MCP) Card: Primary record given to parents
- Immunization Register: Maintained at health facility for all children
- Due List: Monthly list of children due for vaccination
- Session Microplan: Details of session site, date, and target beneficiaries
- Vaccine Stock and Usage Register: Inventory management record
- AEFI Register: Documentation of adverse events
Monitoring and Evaluation
Effective monitoring and evaluation is critical for assessing the performance and impact of the Universal Immunization Programme (UIP). Community health nurses actively participate in these processes to ensure quality immunization services and identify areas for improvement.
Key Indicators
Coverage Indicators:
- Full Immunization Coverage (FIC)
- Antigen-wise coverage
- Dropout rates (DPT1-DPT3, Penta1-Penta3)
- Left-out rate (zero dose children)
Quality Indicators:
- Vaccine wastage rates
- AEFI incidence and reporting rates
- Cold chain equipment functionality
- Session planned vs. held
Impact Indicators:
- Disease incidence rates
- Outbreak occurrence
- Infant and under-five mortality
- Disability-adjusted life years (DALYs) averted
Calculation Methods
Full Immunization Coverage (FIC):
FIC = (Number of children who received all UIP vaccines before 1 year ÷ Total number of surviving infants) × 100
Dropout Rate (DPT1-DPT3):
Dropout Rate = [(DPT1 – DPT3) ÷ DPT1] × 100
Vaccine Wastage Rate:
Wastage Rate = [(Doses issued – Doses administered) ÷ Doses issued] × 100
Session Achievement Rate:
Session Achievement = (Number of sessions held ÷ Number of sessions planned) × 100
Monitoring Methods
| Method | Description | Frequency | Conducted by |
|---|---|---|---|
| Routine Reporting | Standardized reporting formats for coverage, stock, AEFI | Monthly | Community Health Nurses, Health Workers |
| Supportive Supervision | On-site mentoring and problem-solving at session sites | Monthly/Quarterly | Medical Officers, District Immunization Officers |
| Coverage Surveys | Population-based assessment of immunization coverage | Annual/Biennial | External Agencies, Research Institutions |
| Rapid Assessments | Quick assessment of specific aspects like cold chain | As needed | District/State Teams |
| Surveillance Systems | Disease surveillance and AEFI monitoring | Continuous | Integrated Disease Surveillance Programme |
| Data Quality Assessments | Verification of data accuracy and completeness | Biannual | State Monitoring Teams |
eVIN (Electronic Vaccine Intelligence Network)
The Universal Immunization Programme has implemented eVIN to digitize vaccine stocks and storage temperature monitoring:
- Real-time tracking of vaccine stocks and storage temperatures
- Digital record of vaccine movements through smartphone application
- Temperature loggers connected to central server
- SMS alerts for temperature breaches
- Analytics dashboard for program managers
Using Data for Program Improvement
Community health nurses should utilize monitoring data to improve the Universal Immunization Programme through:
- Identifying areas with low coverage or high dropout rates
- Planning targeted interventions for unreached populations
- Addressing quality issues indicated by adverse events
- Optimizing session planning based on attendance data
- Improving vaccine management to reduce wastage
- Advocating for resources based on evidence of need
Comprehensive Evaluation of UIP
Periodic comprehensive evaluations of the Universal Immunization Programme assess broader aspects of program performance:
- Relevance: Alignment with population health needs and national priorities
- Efficiency: Cost-effectiveness and resource utilization
- Effectiveness: Achievement of program objectives and targets
- Impact: Disease reduction and lives saved
- Sustainability: Long-term program viability and funding
- Equity: Fairness in service delivery across different population groups
Challenges and Strategies
Despite the significant achievements of the Universal Immunization Programme (UIP), several challenges persist that affect its implementation and effectiveness. Community health nurses need to understand these challenges and apply appropriate strategies to overcome them.
Operational Challenges
| Challenge | Strategy |
|---|---|
| Cold Chain Maintenance |
|
| Human Resource Constraints |
|
| Vaccine Supply Issues |
|
| Session Planning and Microplanning |
|
Social and Behavioral Challenges
| Challenge | Strategy |
|---|---|
| Vaccine Hesitancy |
|
| Misinformation and Rumors |
|
| Hard-to-Reach Populations |
|
| Awareness and Education |
|
Systemic Challenges
| Challenge | Strategy |
|---|---|
| Data Quality and Use |
|
| Funding and Sustainability |
|
| Integration with Other Health Services |
|
| Surveillance and AEFI Reporting |
|
Innovative Approaches in the Universal Immunization Programme
Several innovations have been introduced to strengthen the UIP and address persistent challenges:
- Mission Indradhanush: Focused campaign to reach unvaccinated and partially vaccinated children
- eVIN (Electronic Vaccine Intelligence Network): Digital platform for real-time vaccine stock management and temperature monitoring
- ANMOL (ANM Online): Tablet-based application for frontline workers to register and track beneficiaries
- IAP-ImmunizeIndia: SMS-based vaccine reminder system
- U-WIN: Digital registry for all vaccination records
- Alternate Vaccine Delivery System: Dedicated mechanism for vaccine transportation to session sites
Role of Community Health Nurses in Overcoming Challenges
Community health nurses can play a pivotal role in addressing Universal Immunization Programme challenges through:
- Building trust with communities through consistent and respectful engagement
- Adapting communication strategies to address local concerns and beliefs
- Working with community volunteers to identify and track defaulters
- Advocating for needed resources and policy changes
- Using data effectively to identify gaps and monitor progress
- Integrating immunization with other maternal and child health services
Global Best Practices
The Universal Immunization Programme (UIP) can benefit from global best practices in immunization service delivery. These examples from different countries provide valuable insights that can be adapted to strengthen India’s UIP.
Bangladesh’s Success Story
Bangladesh has achieved remarkable immunization coverage despite resource constraints:
- Community microplanning with GIS mapping
- Female community health volunteers
- Engagement of religious leaders and institutions
- Doorstep vaccination services
- Urban slum strategies targeting floating populations
Relevant for: High-density areas and mobile populations
Rwanda’s Integrated Approach
Rwanda achieved over 95% coverage through integration and technology:
- Performance-based financing for health facilities
- SMS reminder system for mothers
- Community health worker program (1 per village)
- Electronic immunization registry
- Monthly community health days integrating services
Relevant for: Integration of services and performance monitoring
Thailand’s Quality Focus
Thailand maintains high-quality immunization services through:
- Decentralized vaccine management
- National quality standards for vaccination clinics
- Comprehensive AEFI monitoring system
- Integration with universal health coverage
- School-based vaccination programs
Relevant for: Quality improvement and safety monitoring
Innovative Strategies from Global Programs
| Strategy | Country Example | Application to UIP |
|---|---|---|
| Reaching Every District (RED) Approach | Ethiopia, Tanzania | District-level microplanning, supportive supervision, community engagement, monitoring for action, and planning resources |
| Immunization Champions | Nigeria, Sierra Leone | Identifying community influencers, traditional leaders, and celebrities to advocate for vaccination |
| Mobile Technology Integration | Tanzania (mTrac), Uganda (mHealth) | Real-time data collection, SMS reminders for parents, electronic vaccine registries, and digital reporting systems |
| Immunization Weeks | Latin American countries | Coordinated national campaigns with enhanced visibility, community events, and extended service hours |
| Public-Private Partnerships | Philippines, Kenya | Engaging private practitioners, workplace vaccination programs, and corporate social responsibility initiatives |
| Defaulter Tracking Systems | Zambia, South Africa | Community-based tracking mechanisms, home visits for missed appointments, and incentives for completeness |
Addressing Specific Challenges: Global Examples
Vaccine Hesitancy Solutions
- Canada: Motivational interviewing techniques by healthcare providers to address parents’ concerns
- Australia: No Jab, No Pay policy linking child benefits to immunization status with provisions for genuine medical exemptions
- Malaysia: Multi-religious leader engagement to address faith-based concerns
- UK: Tailored communication approaches based on specific hesitancy types and demographic factors
Hard-to-Reach Population Strategies
- Mongolia: Mobile vaccination teams for nomadic populations using GIS tracking
- Indonesia: Boat clinics for island communities with scheduled visits
- Mexico: Cross-border immunization coordination for migrant populations
- Pakistan: Protected outreach strategies in security-compromised areas with community volunteers
Cold Chain Innovations
Several countries have implemented innovative cold chain solutions applicable to the Universal Immunization Programme:
- Solar Direct Drive (SDD) Refrigerators: Used in Zimbabwe and Uganda to maintain cold chain in areas with unreliable electricity
- Long-range Vaccine Carriers: Developed in Nepal for mountainous regions, keeping vaccines cold for up to 5 days
- Remote Temperature Monitoring: Implemented in Mozambique with SMS alerts for cold chain breaches
- Domestic Refrigerator Adaptation: Low-cost solutions from Philippines to convert regular refrigerators for vaccine storage in underserved areas
Lessons for Community Health Nurses in India
Key takeaways from global best practices for nurses implementing the Universal Immunization Programme:
- Tailor strategies to local contexts and specific community needs
- Integrate immunization with other maternal and child health services for efficiency
- Leverage technology appropriately, even in resource-constrained settings
- Engage community influencers and religious leaders as partners
- Focus on quality and safety alongside coverage targets
- Use data for targeted interventions rather than one-size-fits-all approaches
- Prioritize building trust through consistent and respectful communication
