Screening for Congenital Anomalies
Comprehensive Nursing Education Notes
Table of Contents
- 1. Introduction to Congenital Anomalies
- 2. Fundamentals of Anomaly Screening
- 3. Screening Methods
- 4. Ultrasound Screening
- 5. Genetic Screening Tests
- 6. Common Congenital Anomalies
- 7. Interpretation of Screening Results
- 8. Nursing Considerations
- 9. Ethical Considerations
- 10. Global Best Practices
- 11. References
1. Introduction to Congenital Anomalies
Congenital anomalies, also known as birth defects, are structural or functional abnormalities present at birth that can affect almost any part of the body. They may be identified prenatally, at birth, or later in life. Effective anomaly screening programs are essential for early detection and management of these conditions.
Congenital anomalies affect approximately 3-5% of all births worldwide, making them a significant public health concern. As nursing professionals, understanding the principles and practices of anomaly screening is crucial for providing comprehensive care to expectant mothers and their families.
Definition
Congenital anomalies are structural or functional abnormalities that occur during intrauterine life and can be identified prenatally, at birth, or later in life. They can result from genetic, environmental, or multifactorial causes.
Key Statistics
- Approximately 3-5% of newborns are affected by congenital anomalies globally
- Major congenital anomalies account for about 20% of infant deaths in developed countries
- Up to 70% of severe congenital anomalies can be detected through proper prenatal screening
- Neural tube defects affect approximately 1 in 1,000 pregnancies worldwide
- Congenital heart defects occur in about 8 in 1,000 live births
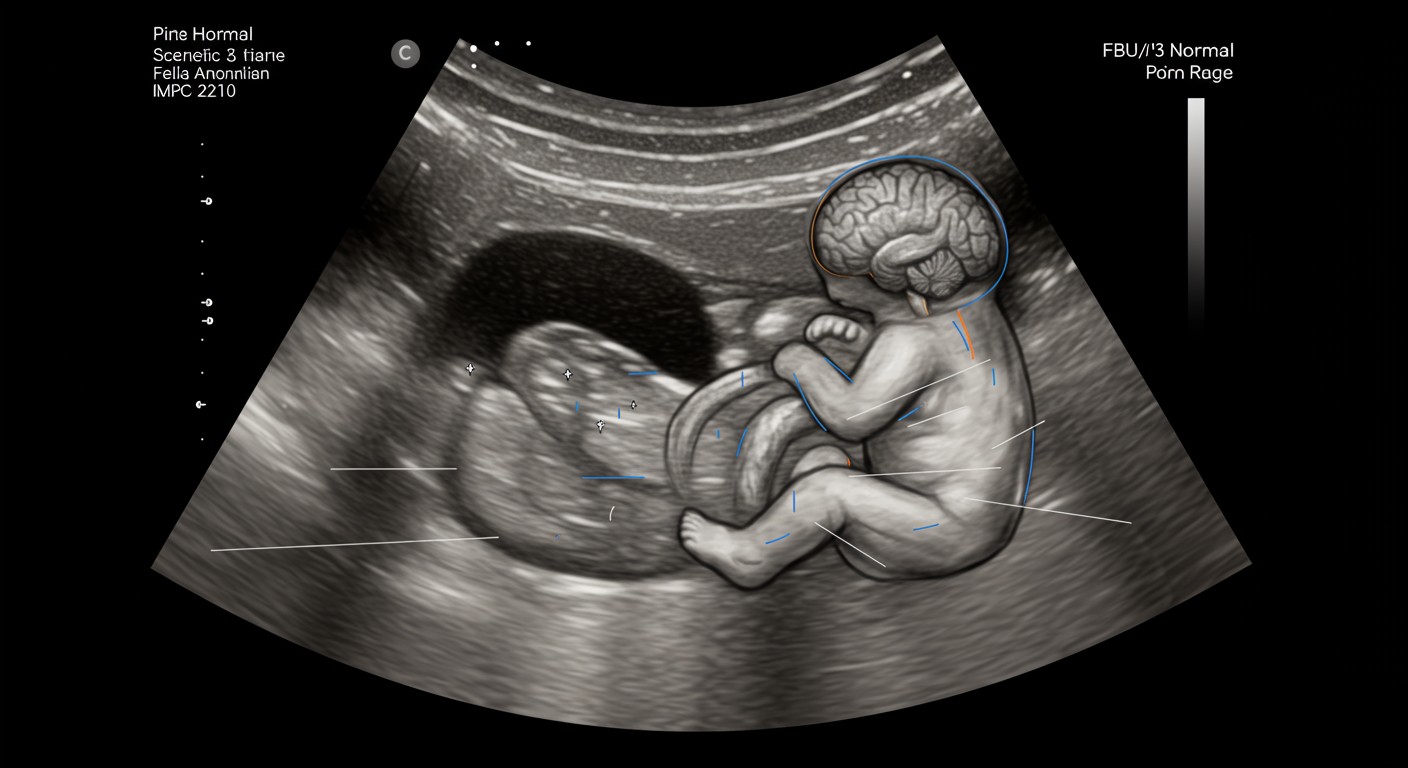
Figure 1: Ultrasound image showing fetal anatomical structures examined during anomaly screening
2. Fundamentals of Anomaly Screening
Anomaly screening encompasses various diagnostic procedures aimed at detecting structural or chromosomal abnormalities in the developing fetus. The primary goals of prenatal anomaly screening are:
- Early detection of congenital anomalies
- Providing information to expectant parents to make informed decisions
- Planning for appropriate perinatal management
- Preparing for specialized care that may be needed after birth
- In some cases, facilitating in-utero interventions
Principles of Effective Anomaly Screening
- Timing: Different screening tests are performed at specific gestational ages for optimal detection
- Integration: Combining multiple screening methods increases detection rates
- Informed consent: Patients must understand the purpose, benefits, limitations, and potential risks of screening
- Interpretation: Results should be interpreted in the context of clinical history and other findings
- Follow-up: Abnormal results require appropriate counseling and follow-up testing
When discussing anomaly screening with patients, emphasize that screening tests identify risk rather than definitively diagnosing conditions. This helps manage expectations and reduces unnecessary anxiety.
Risk Factors for Congenital Anomalies
| Category | Risk Factors | Associated Anomalies |
|---|---|---|
| Maternal Factors | Advanced maternal age (≥35 years), Diabetes mellitus, Obesity, Epilepsy, Phenylketonuria | Chromosomal abnormalities, Neural tube defects, Cardiac defects |
| Lifestyle Factors | Alcohol consumption, Smoking, Illicit drug use | Fetal alcohol spectrum disorders, Growth restriction, Cleft lip/palate |
| Environmental Factors | Radiation exposure, Certain chemicals, High temperatures (hyperthermia) | Various structural defects, Growth restriction |
| Medication Use | Certain anticonvulsants, Retinoids, Thalidomide, ACE inhibitors, Warfarin | Specific patterns of malformation depending on exposure |
| Infections | TORCH infections (Toxoplasmosis, Others, Rubella, Cytomegalovirus, Herpes), Zika virus | Microcephaly, Sensory impairments, Cardiac defects |
| Genetic Factors | Family history of genetic disorders, Consanguinity | Various genetic syndromes and inherited disorders |
Mnemonic: “TORCH” Infections
- T – Toxoplasmosis
- O – Others (Syphilis, Varicella, Parvovirus B19, HIV)
- R – Rubella
- C – Cytomegalovirus (CMV)
- H – Herpes Simplex Virus (HSV)
3. Screening Methods
Prenatal anomaly screening methods can be broadly classified into non-invasive and invasive techniques. Each approach has specific applications, benefits, limitations, and optimal timing during pregnancy.
3.1 Non-Invasive Screening Techniques
Non-invasive screening techniques pose no risk to the pregnancy while providing valuable information about potential congenital anomalies.
| Screening Method | Timing | Detects | Limitations |
|---|---|---|---|
| Ultrasound (First Trimester) | 11-14 weeks | Nuchal translucency, early structural anomalies, viability, gestational age | Cannot detect all anomalies; operator-dependent |
| Ultrasound (Second Trimester) | 18-22 weeks | Major structural anomalies, soft markers for chromosomal abnormalities | Some anomalies may develop later or be subtle |
| Maternal Serum Screening | 15-20 weeks | Risk for trisomies, neural tube defects | Not diagnostic; only provides risk assessment |
| Combined First Trimester Screening | 11-14 weeks | Risk for trisomies, particularly Down syndrome | Higher false-positive rate than NIPT |
| Non-Invasive Prenatal Testing (NIPT) | ≥10 weeks | Common chromosomal abnormalities (trisomies 21, 18, 13) | Does not detect structural anomalies; higher cost |
| Cell-Free DNA Screening | ≥10 weeks | Chromosomal abnormalities, some microdeletions | May not be reliable in certain cases (obesity, twins) |
When discussing non-invasive anomaly screening options with patients, explain that while these tests have high sensitivity, they are screening tools rather than diagnostic tests. Abnormal results typically require confirmation through invasive testing.
3.2 Invasive Screening Procedures
Invasive procedures provide definitive diagnostic information but carry a small risk of pregnancy complications including miscarriage. These are typically offered when non-invasive screening suggests increased risk or when there are specific indications.
| Procedure | Timing | Sample Obtained | Indications | Risks |
|---|---|---|---|---|
| Chorionic Villus Sampling (CVS) | 10-13 weeks | Placental tissue | Abnormal screening results, advanced maternal age, family history of genetic disorders | Miscarriage risk: 0.5-1% |
| Amniocentesis | 15-20 weeks (early amnio: 11-14 weeks) | Amniotic fluid (contains fetal cells) | Abnormal screening results, advanced maternal age, abnormal ultrasound findings | Miscarriage risk: 0.1-0.3% |
| Cordocentesis (PUBS) | ≥18 weeks | Fetal blood from umbilical cord | Severe fetal anemia, infection, certain metabolic disorders | Miscarriage risk: 1-2% |
Tests Performed on Samples from Invasive Procedures
- Karyotyping: Examines chromosome number and structure (results in 10-14 days)
- FISH (Fluorescence In Situ Hybridization): Rapid testing for common chromosomal abnormalities (results in 24-48 hours)
- QF-PCR (Quantitative Fluorescent PCR): Rapid detection of aneuploidies (results in 1-2 days)
- Microarray: Detects submicroscopic chromosomal deletions and duplications
- DNA sequencing: For specific single-gene disorders
- Biochemical testing: For metabolic disorders and neural tube defects (AFP in amniotic fluid)
After invasive procedures, advise patients to monitor for signs that warrant immediate medical attention:
- Vaginal bleeding
- Leaking amniotic fluid
- Severe abdominal pain or cramping
- Fever or chills
- Decreased fetal movement
4. Ultrasound Screening
Ultrasound is the cornerstone of prenatal anomaly screening, providing real-time visualization of fetal anatomy and development. Different types of ultrasound examinations are performed at specific gestational ages to optimize detection of congenital anomalies.
4.1 First Trimester Ultrasound
Performed between 11-14 weeks gestation, first trimester ultrasound is a crucial component of early anomaly screening.
Key Components of First Trimester Ultrasound
- Dating assessment: Crown-rump length measurement to establish gestational age
- Viability assessment: Presence of fetal heartbeat
- Nuchal translucency (NT) measurement: Fluid collection at the back of fetal neck; increased NT is associated with chromosomal abnormalities and cardiac defects
- Nasal bone assessment: Absence may be associated with chromosomal abnormalities
- Basic anatomy check: Skull, brain, spine, abdominal wall, limbs, stomach, bladder
- Placental location: Preliminary assessment
- Multiple pregnancy assessment: Number of fetuses, chorionicity, amnionicity
The optimal time for nuchal translucency measurement is between 11 weeks and 13 weeks 6 days. Encourage patients to attend their first trimester scan within this timeframe for the most accurate anomaly screening results.
4.2 Second Trimester Ultrasound (Anomaly Scan)
The second trimester detailed anomaly screening ultrasound, commonly known as the “anomaly scan,” is typically performed between 18-22 weeks gestation. This is the most comprehensive ultrasound examination during pregnancy, systematically evaluating fetal anatomy.
Head and Brain
- Head shape and cranial bones
- Ventricular system
- Cerebellum and posterior fossa
- Cavum septum pellucidum
- Choroid plexuses
Face
- Orbits and eyes
- Nose and nostrils
- Upper lip (to exclude cleft lip)
- Profile and facial features
Neck and Spine
- Cervical, thoracic, lumbar vertebrae
- Skin covering (to exclude neural tube defects)
- Normal curvature
Chest
- Lung texture
- Diaphragm contour
- Ribs
Heart
- Four-chamber view
- Outflow tracts
- Cardiac rhythm
- Size and position
Abdomen
- Stomach position
- Liver and bowel
- Kidneys and bladder
- Abdominal wall (to exclude defects)
- Cord insertion
Limbs
- Arms, hands, legs, feet
- All long bones
- Digits (fingers and toes)
- Movement and position
Genitalia
- Gender determination (if requested)
- Normal external genitalia
Mnemonic: “ABCDEFGH” for Anomaly Scan
- A – Amniotic fluid volume
- B – Brain structures and Bones
- C – Cardiac views and Chest
- D – Diaphragm and Digestive structures
- E – Extremities (limbs)
- F – Face and Fetal position
- G – Genitalia and Growth parameters
- H – Heart and Head measurements
4.3 Third Trimester Ultrasound
Third trimester ultrasounds are not routinely performed as part of anomaly screening but may be indicated in certain situations:
- Assessment of fetal growth
- Evaluation of placental position and function
- Amniotic fluid volume assessment
- Follow-up of previously detected anomalies
- Evaluation of fetal well-being
- Detection of late-onset anomalies
Some congenital anomalies, particularly certain cardiac defects and obstructive uropathies, may not become apparent until the third trimester. Maintain vigilance for signs of fetal compromise even after normal second trimester anomaly screening.
Advanced Ultrasound Techniques in Anomaly Screening
- 3D/4D Ultrasound: Provides detailed surface rendering and may improve detection of facial anomalies, skeletal dysplasias, and neural tube defects
- Doppler Ultrasound: Evaluates blood flow in fetal vessels; particularly useful for assessing cardiac function and placental circulation
- Fetal Echocardiography: Detailed examination of the fetal heart; indicated when cardiac anomalies are suspected
- Neurosonography: Detailed assessment of the fetal central nervous system using special views and higher-resolution transducers
5. Genetic Screening Tests
Genetic screening tests complement ultrasound in comprehensive anomaly screening programs. These tests analyze maternal blood samples to assess the risk of chromosomal abnormalities and, in some cases, genetic disorders.
| Test | Timing | Methodology | Detects | Detection Rate |
|---|---|---|---|---|
| Combined First Trimester Screening | 11-14 weeks | NT ultrasound + serum markers (PAPP-A, free β-hCG) | Trisomy 21, 18, 13 risk | 85-90% (T21) |
| Quadruple Screen | 15-20 weeks | Serum markers (AFP, hCG, uE3, inhibin A) | Trisomy 21, 18, NTDs | 80% (T21) |
| Integrated Screen | 11-14 weeks + 15-20 weeks | NT + first and second trimester serum markers | Trisomy 21, 18, NTDs | 94-96% (T21) |
| Cell-Free DNA (NIPT) | ≥10 weeks | Analysis of cell-free fetal DNA in maternal blood | Trisomy 21, 18, 13, sex chromosome abnormalities | 99% (T21) |
| Expanded NIPT | ≥10 weeks | Advanced cell-free DNA analysis | Above + selected microdeletions | Varies by condition |
| Carrier Screening | Pre-conception or early pregnancy | DNA analysis | Recessive genetic disorders (CF, SMA, etc.) | Varies by condition |
Key Serum Markers Used in Anomaly Screening
- PAPP-A (Pregnancy-Associated Plasma Protein A): Low levels associated with trisomies
- Free β-hCG (human Chorionic Gonadotropin): High in trisomy 21, low in trisomy 18
- AFP (Alpha-Fetoprotein): Elevated in open neural tube defects, low in trisomy 21
- uE3 (unconjugated Estriol): Low levels associated with trisomies
- Inhibin A: Elevated in trisomy 21
When discussing NIPT (Non-Invasive Prenatal Testing) with patients, emphasize its high sensitivity and specificity for common chromosomal abnormalities, but explain that it does not screen for all genetic disorders or structural anomalies. Comprehensive anomaly screening should still include detailed ultrasound examination.
Limitations of Genetic Screening
- False positives and false negatives: All screening tests have inherent limitations in accuracy
- Limited scope: Most genetic screening tests only target common chromosomal abnormalities
- Technical failures: Some tests may fail to provide results due to insufficient fetal fraction or technical issues
- Unexpected findings: Tests may identify maternal chromosomal abnormalities or microdeletions of uncertain significance
- Multiple gestations: Some tests have limited accuracy or are not validated for twin or higher-order pregnancies
6. Common Congenital Anomalies
Understanding the typical presentations and anomaly screening findings for common congenital anomalies helps nursing professionals provide informed guidance and support to patients. This section outlines key features and screening considerations for major categories of congenital anomalies.
6.1 Neural Tube Defects
Neural tube defects (NTDs) result from incomplete closure of the neural tube during early embryonic development (3-4 weeks after conception). Anomaly screening programs have significantly improved early detection of these conditions.
| Type of NTD | Description | Screening Findings |
|---|---|---|
| Anencephaly | Absence of major portion of brain, skull, and scalp | Absent cranial vault; easily detected on early ultrasound; elevated maternal serum AFP |
| Spina Bifida | Incomplete closure of spine with meninges and/or spinal cord exposure | Abnormal spine appearance; “lemon” and “banana” signs in brain; elevated maternal serum AFP |
| Encephalocele | Protrusion of brain tissue and meninges through skull defect | Visible cystic mass on cranium; elevated maternal serum AFP |
Cranial Signs of Spina Bifida on Ultrasound
- Lemon sign: Frontal bone scalloping giving the head a lemon-like appearance
- Banana sign: Abnormal curved cerebellum resembling a banana
- Ventriculomegaly: Dilated ventricles in the brain
6.2 Cardiac Anomalies
Congenital heart defects (CHDs) are the most common type of birth defect, affecting approximately 1% of live births. Anomaly screening for cardiac defects is a critical component of the mid-trimester anomaly scan.
Septal Defects
- Ventricular Septal Defect (VSD): Opening in ventricular septum
- Atrial Septal Defect (ASD): Opening in atrial septum
- Atrioventricular Septal Defect (AVSD): Defect in central heart structures
Outflow Tract Anomalies
- Tetralogy of Fallot: VSD, pulmonary stenosis, overriding aorta, right ventricular hypertrophy
- Transposition of Great Arteries: Aorta connects to right ventricle; pulmonary artery to left
Valve Anomalies
- Pulmonary/Aortic Stenosis: Narrowing of valves
- Hypoplastic Left Heart: Underdevelopment of left ventricle and aorta
- Ebstein Anomaly: Displacement of tricuspid valve
During anomaly screening, the four-chamber view of the heart is essential but detects only about 60% of major cardiac defects. The addition of outflow tract views increases detection rates to approximately 90%. When discussing cardiac screening with patients, emphasize the importance of specialized fetal echocardiography when indicated by risk factors or suspicious findings.
6.3 Gastrointestinal Anomalies
Gastrointestinal anomalies affect approximately 1 in 2,000 births and are often detectable during routine anomaly screening.
| Anomaly | Description | Ultrasound Findings |
|---|---|---|
| Gastroschisis | Abdominal wall defect with intestinal herniation; no membrane covering | Free-floating intestines in amniotic fluid; defect usually to right of umbilicus |
| Omphalocele | Midline abdominal defect with membrane-covered herniation of abdominal contents | Membrane-covered mass at umbilicus; often contains liver |
| Duodenal Atresia | Complete obstruction of the duodenum | “Double bubble” sign (dilated stomach and proximal duodenum) |
| Esophageal Atresia | Incomplete formation of esophagus | Small/absent stomach bubble; polyhydramnios |
| Diaphragmatic Hernia | Defect in diaphragm allowing abdominal contents into chest | Abdominal organs in thorax; mediastinal shift; small abdominal cavity |
Mnemonic: “VACTERL” Association
A non-random association of defects that may be identified during anomaly screening:
- V – Vertebral anomalies
- A – Anal atresia
- C – Cardiac defects
- T – Tracheo-Esophageal fistula
- E – Esophageal atresia
- R – Renal anomalies
- L – Limb defects
6.4 Genitourinary Anomalies
Genitourinary anomalies affect approximately 1 in 500 newborns. Anomaly screening identifies many of these conditions, allowing for appropriate planning and management.
Renal Anomalies
- Renal agenesis: Absence of one or both kidneys
- Multicystic dysplastic kidney: Non-functioning kidney with multiple cysts
- Polycystic kidney disease: Multiple bilateral renal cysts
- Horseshoe kidney: Fusion of lower poles of kidneys
Urinary Tract Anomalies
- Hydronephrosis: Dilation of renal pelvis
- Ureteropelvic junction obstruction: Blockage where kidney meets ureter
- Posterior urethral valves: Obstructive membrane in male urethra
- Bladder exstrophy: Exposed bladder due to abdominal wall defect
Potter Sequence
Bilateral renal agenesis leads to Potter sequence, characterized by:
- Oligohydramnios (severe reduction in amniotic fluid)
- Pulmonary hypoplasia
- Characteristic facial features
- Limb positioning abnormalities
Early detection through anomaly screening allows for appropriate counseling regarding the poor prognosis of this condition.
6.5 Skeletal Anomalies
Skeletal dysplasias and anomalies encompass a diverse group of conditions affecting bone and cartilage development. Anomaly screening can detect many skeletal anomalies, particularly during the second trimester scan.
| Anomaly | Description | Ultrasound Findings |
|---|---|---|
| Thanatophoric Dysplasia | Severe skeletal dysplasia, usually lethal | Very short limbs, narrow chest, large head, cloverleaf skull (in type II) |
| Achondroplasia | Most common form of dwarfism | Short limbs, normal trunk, macrocephaly with frontal bossing |
| Osteogenesis Imperfecta | Brittle bone disease | Multiple fractures, bone deformities, reduced mineralization |
| Club Foot (Talipes) | Foot abnormally twisted, often inward | Abnormal position of foot relative to lower leg |
| Polydactyly | Extra digits (fingers or toes) | More than 5 digits on hand or foot |
When skeletal anomalies are detected during anomaly screening, consider the possibility of associated syndromes or chromosomal abnormalities. Multiple skeletal findings often indicate a syndromic condition requiring comprehensive genetic evaluation.
7. Interpretation of Screening Results
Accurate interpretation of anomaly screening results is essential for appropriate patient counseling and management. This section discusses key considerations in result interpretation and subsequent steps.
Understanding Screening Test Performance
| Parameter | Definition | Relevance to Anomaly Screening |
|---|---|---|
| Sensitivity | Proportion of affected individuals correctly identified as positive | Higher sensitivity means fewer false negatives (missed anomalies) |
| Specificity | Proportion of unaffected individuals correctly identified as negative | Higher specificity means fewer false positives |
| Positive Predictive Value (PPV) | Probability that individuals with positive test truly have the condition | Affected by prevalence; lower for rare conditions |
| Negative Predictive Value (NPV) | Probability that individuals with negative test truly don’t have the condition | Generally high for well-designed screening programs |
| Detection Rate | Proportion of affected cases detected by screening | Key measure of screening program effectiveness |
| False Positive Rate | Proportion of unaffected individuals with positive results | Impacts number of unnecessary invasive procedures |
When explaining anomaly screening results to patients, use absolute numbers rather than percentages when possible. For example, “1 in 100 chance” is often better understood than “1% risk.” Visual aids can also help patients understand probability concepts.
Soft Markers on Ultrasound
“Soft markers” are ultrasound findings that may be associated with increased risk of chromosomal abnormalities but are not structural anomalies themselves. Their significance in anomaly screening depends on whether they appear in isolation or with other findings.
| Soft Marker | Description | Significance |
|---|---|---|
| Nuchal Fold Thickening | Increased thickness of skin at back of fetal neck | Associated with Down syndrome; significant marker |
| Echogenic Intracardiac Focus | Bright spot in fetal heart | Weak association with aneuploidy when isolated |
| Echogenic Bowel | Intestines appear as bright as bone | Associated with chromosomal abnormalities, CF, infections |
| Mild Ventriculomegaly | Slight enlargement of cerebral ventricles | May indicate chromosomal or CNS abnormalities |
| Renal Pyelectasis | Mild dilation of renal pelvis | Weak association with aneuploidy when isolated |
| Short Femur/Humerus | Limb length below expected for gestational age | Associated with growth restriction, skeletal dysplasias |
| Single Umbilical Artery | Only one artery in umbilical cord instead of two | Associated with renal, cardiac anomalies |
Managing Abnormal Results
When anomaly screening identifies potential abnormalities, a structured approach to follow-up is essential:
Confirm finding with careful reassessment; document details including measurements and images
Arrange prompt referral to maternal-fetal medicine specialist or fetal medicine unit for detailed evaluation
Comprehensive ultrasound assessment by specialist; additional imaging (MRI) if indicated
Discussion of findings, implications, and options for diagnostic testing
Offer invasive testing (amniocentesis, CVS) for definitive diagnosis when indicated
Involve relevant specialists (pediatric surgery, cardiology, neurology, etc.) based on findings
Develop comprehensive plan for remainder of pregnancy, delivery, and neonatal care
Provide continuous emotional support, information, and connection to appropriate resources
When discussing abnormal anomaly screening results, avoid using definitive language until diagnostic confirmation is available. Use phrases like “increased risk” or “concerning finding that requires further evaluation” rather than stating a diagnosis prematurely.
8. Nursing Considerations
Nurses play a pivotal role in prenatal anomaly screening programs, providing education, support, and coordination of care. This section outlines key nursing considerations throughout the screening process.
Pre-Screening Education and Counseling
- Informed decision-making: Discuss available screening options, their purpose, benefits, limitations, and potential implications
- Timing considerations: Explain optimal timing for different screening tests and importance of adhering to timeframes
- Voluntary nature: Emphasize that anomaly screening is optional and respect patient choices
- Preparation guidance: Provide instructions for test preparation (e.g., full bladder for early pregnancy ultrasound)
- Expectation management: Explain what to expect during procedures, including duration and potential discomfort
Key Points for Patient Education on Anomaly Screening
- The difference between screening and diagnostic tests
- The meaning of positive and negative results
- The possibility of false positives and false negatives
- The limitations of screening (what conditions can and cannot be detected)
- The follow-up process if abnormal results are found
- The timeline for receiving results
During Screening Procedures
- Comfort measures: Position patient comfortably and provide emotional support during procedures
- Communication: Facilitate communication between patient and healthcare providers performing the screening
- Observation: Monitor for adverse reactions during invasive procedures
- Documentation: Ensure accurate documentation of procedures, findings, and patient responses
During ultrasound anomaly screening, patients often have high expectations about seeing their baby and may not fully comprehend the medical purpose of the examination. Gently remind patients about the screening nature of the procedure while acknowledging the emotional significance of seeing their baby.
Post-Screening Support and Follow-Up
- Results communication: Provide results in clear, understandable language with appropriate sensitivity
- Emotional support: Recognize and address emotional responses to abnormal results
- Referral coordination: Facilitate timely referrals to specialists when indicated
- Resource provision: Connect patients with appropriate resources, support groups, and services
- Continuity of care: Ensure smooth transition between care providers and settings
Communication Strategies for Delivering Abnormal Results
- Provide information in a private setting with adequate time for discussion
- Use clear, non-technical language while avoiding euphemisms
- Present information in manageable portions, checking understanding throughout
- Acknowledge and validate emotional responses
- Provide written information to supplement verbal explanations
- Outline next steps clearly and concretely
- Offer presence during subsequent consultations if desired
When caring for patients after receiving abnormal anomaly screening results, be vigilant for signs of acute stress reactions or psychological distress. These may include persistent anxiety, insomnia, withdrawal, difficulty concentrating, or expressions of guilt. Prompt referral to mental health resources may be necessary.
Documentation Requirements
Proper documentation is essential in anomaly screening programs to ensure continuity of care and medicolegal protection. Document:
- Information provided to patient about screening options
- Informed consent for screening procedures
- Screening tests performed and dates
- Results of screening tests
- Communication of results to patient
- Patient’s understanding and response to results
- Follow-up plans and referrals made
- Resources provided to patient
9. Ethical Considerations
Prenatal anomaly screening raises important ethical considerations that nursing professionals should be prepared to address sensitively. This section explores key ethical dimensions of screening programs.
Informed Consent
Respect for patient autonomy requires comprehensive information about:
- Nature and purpose of screening
- Limitations and accuracy of tests
- Potential findings and implications
- Available options following results
- Right to decline screening
Non-Directive Counseling
Healthcare providers should:
- Present information without bias
- Avoid imposing personal values
- Support patient’s autonomous decision-making
- Respect cultural and religious perspectives
Equity and Access
Ethical screening programs should address:
- Equitable access across socioeconomic groups
- Cultural sensitivity in delivery
- Language barriers and health literacy
- Financial barriers to screening and follow-up
Privacy and Confidentiality
Protection of sensitive information through:
- Secure handling of test results
- Appropriate disclosure policies
- Respect for patients’ information preferences
- Compliance with relevant regulations
Ethical Dilemmas in Anomaly Screening
- Incidental findings: Managing unexpected findings of uncertain significance
- Late diagnosis: Balancing autonomy with limited options when anomalies are detected late in pregnancy
- Resource allocation: Determining appropriate allocation of limited healthcare resources for screening programs
- Variant interpretation: Addressing findings with variable expressivity or uncertain pathogenicity
- Societal implications: Considering broader social messages about disability and human value
When discussing anomaly screening with patients from diverse cultural backgrounds, be aware that cultural perspectives may significantly influence decision-making. Use cultural brokers or interpreters when appropriate, and explore the patient’s cultural context and values before providing information about screening options.
10. Global Best Practices
Best practices in prenatal anomaly screening vary globally based on healthcare systems, resources, and cultural contexts. This section highlights exemplary approaches and emerging trends.
Integrated Screening Programs
Several countries have implemented comprehensive, integrated anomaly screening programs that combine multiple modalities for optimal detection:
| Country/Region | Program Features | Noteworthy Elements |
|---|---|---|
| United Kingdom | NHS Fetal Anomaly Screening Programme (FASP) | Standardized protocols; quality assurance framework; national database for outcomes tracking |
| Denmark | Universal combined first trimester screening | High uptake (>90%); centralized quality control; comprehensive follow-up pathways |
| Australia | State-based programs with national guidelines | Rural and remote access initiatives; telemedicine support for isolated communities |
| Singapore | Integrated screening pathway | High-quality imaging standards; efficient referral networks; comprehensive genetic counseling |
Emerging Technologies and Approaches
- Cell-free DNA expansion: Expanding NIPT to detect microdeletions and single-gene disorders
- Artificial intelligence: Machine learning algorithms to improve detection of subtle anomalies on ultrasound
- Telemedicine: Remote expert review of imaging from underserved areas
- Patient-centered decision aids: Interactive tools to facilitate informed decision-making
- Expanded carrier screening: Genome-based carrier testing for hundreds of recessive conditions
Quality Indicators for Anomaly Screening Programs
- Detection rates: Proportion of cases identified prenatally compared to total cases
- False positive rates: Proportion of unaffected pregnancies with positive screening results
- Timely reporting: Results provided within defined timeframes
- Follow-up completion: Proportion of abnormal results with appropriate follow-up
- Patient satisfaction: Measures of information adequacy and support
- Workforce competency: Ongoing training and certification of providers
- Equipment standards: Regular calibration and upgrading of imaging equipment
World Health Organization Recommendations
The WHO recommends that countries implement anomaly screening programs that are:
- Equitable and accessible to all population segments
- Integrated within existing maternal health services
- Supported by appropriate counseling services
- Linked to intervention and treatment pathways
- Subject to quality assurance mechanisms
- Monitored for outcomes and impact
11. References
- American College of Obstetricians and Gynecologists. (2020). Practice Bulletin No. 226: Screening for Fetal Chromosomal Abnormalities. Obstetrics & Gynecology, 136(4), e48-e69.
- International Society of Ultrasound in Obstetrics and Gynecology. (2021). ISUOG Practice Guidelines: Performance of the routine mid-trimester fetal ultrasound scan. Ultrasound in Obstetrics & Gynecology, 57(5), 797-811.
- National Institute for Health and Care Excellence. (2021). Antenatal care (NG201). Retrieved from https://www.nice.org.uk/guidance/ng201
- Salomon, L. J., Alfirevic, Z., Berghella, V., Bilardo, C., Hernandez-Andrade, E., Johnsen, S. L., et al. (2011). Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound in Obstetrics & Gynecology, 37(1), 116-126.
- Gregg, A. R., Skotko, B. G., Benkendorf, J. L., Monaghan, K. G., Bajaj, K., Best, R. G., et al. (2016). Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genetics in Medicine, 18(10), 1056-1065.
- World Health Organization. (2016). WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization.
- Schmid, M., & Klaritsch, P. (2014). Fetal cardiac screening: what is the evidence?. Ultraschall in der Medizin, 35(6), 481-484.
- Hui, L., & Bianchi, D. W. (2017). Noninvasive prenatal DNA testing: the vanguard of genomic medicine. Annual Review of Medicine, 68, 459-472.
- Moorthie, S., Blencowe, H., Darlison, M. W., Lawn, J., Morris, J. K., Modell, B., et al. (2018). Estimating the birth prevalence and pregnancy outcomes of congenital malformations worldwide. Journal of Community Genetics, 9(4), 387-396.
- Chitty, L. S., & Hui, L. (2019). Non-invasive prenatal testing for aneuploidy—current status and future prospects. Prenatal Diagnosis, 39(10), 827-836.
Additional Resources for Nurses
- International Society of Nurses in Genetics: https://www.isong.org/
- Society of Maternal-Fetal Medicine: https://www.smfm.org/
- March of Dimes: https://www.marchofdimes.org/
- National Society of Genetic Counselors: https://www.nsgc.org/
