Comprehensive Nursing Notes
Babies Born to Rh-Negative Mothers
A detailed guide to understanding, assessing, and caring for newborns at risk of Rh incompatibility
Introduction to Rh Incompatibility
Approximately 15% of the Caucasian population, 8% of African Americans, and 1% of Asian and Native Americans have Rh-negative blood types. When an Rh-negative mother carries an Rh-positive fetus, there is potential for an incompatibility that can lead to hemolytic disease of the fetus and newborn (HDFN), a condition that can range from mild to life-threatening.
Rh incompatibility occurs when a pregnant woman has Rh-negative blood and the baby she is carrying has Rh-positive blood. This blood type mismatch can cause the mother’s immune system to identify the baby’s Rh-positive red blood cells as foreign, leading to the production of antibodies against these cells. These maternal antibodies can cross the placenta and destroy the baby’s red blood cells, causing anemia, jaundice, and in severe cases, hydrops fetalis or even death.
As nursing professionals, understanding the pathophysiology, risk factors, and management strategies for babies born to Rh-negative mothers is crucial for providing optimal care and preventing adverse outcomes. These comprehensive notes will guide you through all aspects of care for these infants, from pathophysiology to prevention strategies.
Pathophysiology of Rh Incompatibility
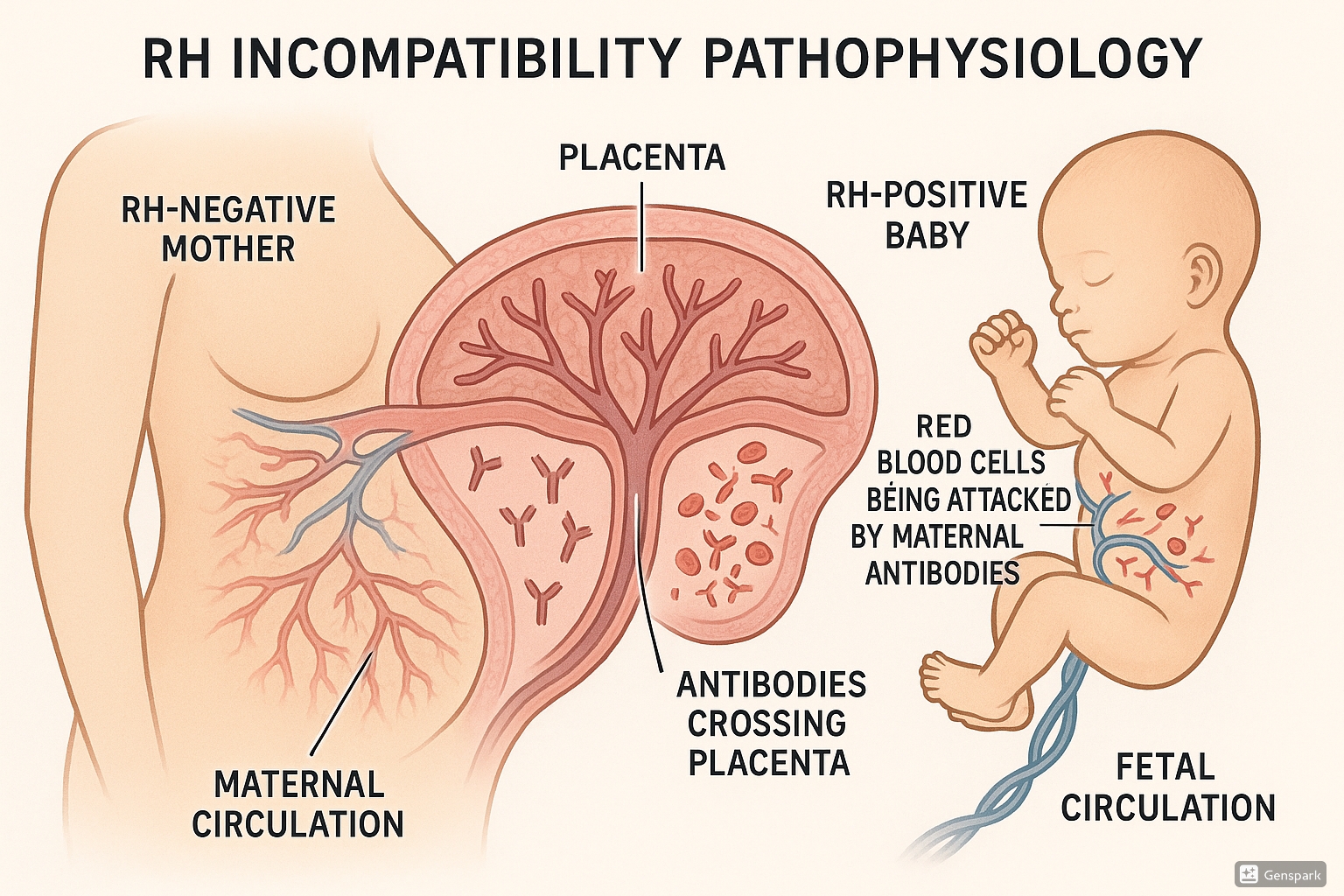
Figure 1: Pathophysiology of Rh incompatibility showing antibodies from an Rh-negative mother crossing the placenta to attack Rh-positive fetal red blood cells
The Rh Factor
The Rh factor is a protein found on the surface of red blood cells (RBCs). Individuals who have this protein are Rh-positive (Rh+), while those without it are Rh-negative (Rh-). The Rh factor is determined genetically, and the Rh-positive trait is dominant over Rh-negative.
Sensitization Process
Sensitization, or isoimmunization, is the process by which an Rh-negative mother develops antibodies against the Rh antigen. This typically occurs in one of the following situations:
Initial Exposure
Small amounts of fetal Rh-positive blood cells enter the maternal circulation, usually during delivery, miscarriage, abortion, ectopic pregnancy, or after invasive procedures such as amniocentesis or chorionic villus sampling.
Primary Immune Response
The mother’s immune system identifies the Rh antigen as foreign and begins producing antibodies, primarily immunoglobulin M (IgM), which are too large to cross the placenta. This initial sensitization typically doesn’t affect the current pregnancy.
Secondary Immune Response
In subsequent pregnancies with an Rh-positive fetus, the mother’s immune system mounts a stronger and faster response, producing immunoglobulin G (IgG) antibodies.
Antibody Transfer
These IgG antibodies can cross the placenta and attach to the Rh antigens on the fetal RBCs, marking them for destruction by the fetal immune system’s macrophages.
Consequences of Hemolysis
The destruction of fetal RBCs (hemolysis) leads to:
- Anemia: Decreased oxygen-carrying capacity due to reduced circulating RBCs
- Hyperbilirubinemia: Elevated levels of bilirubin (a breakdown product of hemoglobin) in the blood
- Jaundice: Yellowing of the skin and eyes due to hyperbilirubinemia
- Hepatosplenomegaly: Enlargement of the liver and spleen as they work to filter the damaged RBCs
- Hydrops fetalis: Severe edema and fluid accumulation in the fetal tissues and body cavities in advanced cases
Important Note on Rh Incompatibility
The severity of Rh incompatibility typically increases with each subsequent pregnancy involving an Rh-positive fetus, as the maternal immune response becomes stronger. This emphasizes the critical importance of preventive measures like RhoGAM administration.
Maternal-Fetal Blood Types and Risk Assessment
Blood Type Inheritance and Risk Patterns
The risk of Rh incompatibility depends on the blood types of both parents and the inheritance pattern of the Rh factor, which follows Mendelian genetics.
| Father’s Blood Type | Mother’s Blood Type | Risk of Rh Incompatibility | Explanation |
|---|---|---|---|
| Rh-positive (homozygous) | Rh-negative | 100% | All children will be Rh-positive |
| Rh-positive (heterozygous) | Rh-negative | 50% | 50% chance of Rh-positive child |
| Rh-negative | Rh-negative | 0% | All children will be Rh-negative |
| Any Rh type | Rh-positive | 0% | Mother’s Rh-positive status protects against sensitization |
Risk Factors for Rh Sensitization
- Previous pregnancies with Rh-positive fetuses
- Previous miscarriages or induced abortions
- Ectopic pregnancies
- Invasive prenatal procedures (amniocentesis, chorionic villus sampling)
- Antepartum hemorrhage (placental abruption, placenta previa)
- Manual removal of the placenta
- External cephalic version (turning of the fetus from breech to vertex position)
- Abdominal trauma during pregnancy
- Blood transfusion with Rh-positive blood
Other Blood Group Incompatibilities
While Rh incompatibility is the most well-known cause of hemolytic disease, ABO incompatibility can also occur, particularly when:
- Mother has blood type O
- Baby has blood type A, B, or AB
Unlike Rh incompatibility, ABO incompatibility can affect first pregnancies because naturally occurring anti-A and anti-B antibodies already exist in type O maternal blood. However, ABO incompatibility is typically less severe than Rh incompatibility due to the weaker expression of A and B antigens on fetal red blood cells and the presence of these antigens on many other cell types.
Prevention of Rh Sensitization
Rho(D) Immune Globulin (RhoGAM)
RhoGAM is a blood product made from human plasma containing anti-Rh(D) antibodies. It works by binding to any fetal Rh-positive blood cells that have entered the mother’s circulation, preventing her immune system from recognizing them and producing antibodies.
How RhoGAM Works
When administered to an Rh-negative mother, RhoGAM binds to any fetal Rh-positive red blood cells in her bloodstream, masking them from her immune system. This prevents sensitization and the development of anti-Rh antibodies that could harm a future Rh-positive fetus.
RhoGAM Administration Timeline
| Timing | Dosage | Indication | Purpose |
|---|---|---|---|
| 28 weeks gestation | 300 mcg (standard dose) | Routine antenatal prophylaxis | Prevents sensitization during third trimester |
| Within 72 hours of delivery | 300 mcg (standard dose) | If baby is Rh-positive | Prevents sensitization from delivery-related bleeding |
| After miscarriage, abortion, or ectopic pregnancy | 50 mcg (MICRhoGAM) if <12 weeks 300 mcg if >12 weeks |
Following pregnancy loss | Prevents sensitization from fetal-maternal hemorrhage |
| After invasive procedures (amniocentesis, CVS) | 300 mcg | Following procedure | Prevents sensitization from procedure-related bleeding |
| After abdominal trauma or antepartum bleeding | 300 mcg | Following event | Prevents sensitization from potential fetal-maternal hemorrhage |
Critical Timing
RhoGAM must be administered within 72 hours of a potentially sensitizing event for maximum effectiveness. However, some benefit may still be achieved even if given up to 10 days after exposure.
RhoGAM Effectiveness
When properly administered, RhoGAM is approximately 99% effective in preventing Rh sensitization. Before the introduction of RhoGAM in the late 1960s, hemolytic disease of the newborn affected approximately 14% of pregnancies in Rh-negative women. Today, with proper prophylaxis, the incidence has dropped to less than 0.1%.
Contraindications and Considerations
- RhoGAM is not administered to women who:
- Are Rh-positive
- Have already developed anti-Rh antibodies (already sensitized)
- Have a history of severe allergic reaction to human immune globulin products
- RhoGAM is considered safe during pregnancy and breastfeeding
- RhoGAM does not protect against other blood group incompatibilities (ABO, Kell, etc.)
Assessment and Diagnosis of Hemolytic Disease of the Newborn
Maternal Assessment
Assessment of the mother with Rh-negative blood type includes:
- Blood type and antibody screening: Performed at first prenatal visit
- Antibody titer monitoring: If sensitized, antibody titers are monitored throughout pregnancy
- Indirect Coombs test: Detects anti-Rh antibodies in maternal serum
- Obstetric history: Previous pregnancies, miscarriages, abortions, blood transfusions
- RhoGAM administration history: Documentation of previous prophylaxis
Fetal Assessment
For pregnancies at risk of Rh incompatibility, fetal monitoring includes:
| Assessment Technique | Purpose | Timing/Frequency | Findings in Hemolytic Disease |
|---|---|---|---|
| Middle Cerebral Artery (MCA) Doppler | Assess for fetal anemia | Every 1-2 weeks from 18 weeks if mother is sensitized | Increased peak systolic velocity indicating anemia |
| Ultrasound | Evaluate for signs of hydrops fetalis | Every 1-2 weeks in high-risk cases | Ascites, pleural/pericardial effusion, skin edema, polyhydramnios |
| Amniocentesis | Measure amniotic fluid bilirubin levels (ΔOD450) | As indicated by antibody titers or Doppler | Elevated bilirubin levels indicating hemolysis |
| Percutaneous umbilical blood sampling (PUBS) | Direct measurement of fetal hemoglobin and hematocrit | When severe anemia is suspected | Decreased hemoglobin/hematocrit, elevated reticulocyte count |
Newborn Assessment
Assessment of the newborn born to an Rh-negative mother includes:
- Blood type and Rh factor determination: From cord blood at birth
- Direct Coombs test: Detects maternal antibodies bound to infant’s RBCs
- Complete blood count (CBC): Evaluates for anemia and reticulocytosis
- Bilirubin levels: Total and direct (conjugated) bilirubin
- Physical assessment: For signs of jaundice, pallor, hepatosplenomegaly
- Vital signs: Monitoring for tachycardia, respiratory distress
Clinical Manifestations
The clinical presentation of hemolytic disease in newborns varies depending on the severity of the condition. Manifestations range from mild jaundice to severe hydrops fetalis.
Mild to Moderate Hemolytic Disease
- Jaundice appearing within the first 24 hours of life
- Mild to moderate pallor
- Normal or slightly elevated heart rate
- Normal activity and feeding
- Mild hepatosplenomegaly
Severe Hemolytic Disease
- Marked pallor at birth
- Rapidly developing intense jaundice
- Pronounced hepatosplenomegaly
- Tachycardia and tachypnea
- Lethargy and poor feeding
- Petechiae or purpura (due to thrombocytopenia)
- Edema
Hydrops Fetalis
The most severe form of hemolytic disease, hydrops fetalis, presents with:
- Generalized edema (anasarca)
- Ascites
- Pleural and/or pericardial effusions
- Severe anemia
- Enlarged heart
- Respiratory distress
- Hepatosplenomegaly
Clinical Progression of Jaundice in Neonates
Jaundice in neonates typically follows a cephalocaudal progression (head to toe). This can help assess the severity:
- Face only: Mild (bilirubin approximately 5-7 mg/dL)
- Upper chest: Moderate (bilirubin approximately 8-10 mg/dL)
- Abdomen and thighs: More severe (bilirubin approximately 11-15 mg/dL)
- Arms and lower legs: Severe (bilirubin >15 mg/dL)
- Palms and soles: Very severe (bilirubin >20 mg/dL)
Laboratory Findings
Laboratory tests are essential for diagnosing and monitoring hemolytic disease of the newborn. Key findings include:
| Laboratory Test | Normal Values | Findings in Hemolytic Disease | Clinical Significance |
|---|---|---|---|
| Direct Coombs Test (DAT) | Negative | Positive (strongly positive in Rh HDN, weakly positive in ABO HDN) | Confirms presence of maternal antibodies on infant’s RBCs |
| Hemoglobin (Hb) | 14-20 g/dL | Decreased (<14 g/dL) | Indicates severity of anemia |
| Hematocrit (Hct) | 45-65% | Decreased (<45%) | Indicates severity of anemia |
| Reticulocyte Count | 3-7% | Increased (may be 10-40%) | Indicates bone marrow response to anemia |
| Total Bilirubin | <5 mg/dL at birth <12 mg/dL by day 3 |
Elevated, often rising rapidly | Indicates degree of hemolysis |
| Direct (Conjugated) Bilirubin | <1.5 mg/dL or <20% of total | Usually normal | Elevated direct bilirubin suggests other causes |
| Peripheral Blood Smear | Normal RBC morphology | Nucleated RBCs, spherocytes, polychromasia | Indicates active hemolysis and compensatory erythropoiesis |
| Albumin | 3.5-5.0 g/dL | May be decreased in severe cases | Bilirubin binding capacity affects risk of kernicterus |
Key Laboratory Distinction
In Rh incompatibility, the Direct Coombs test is typically strongly positive, while in ABO incompatibility it may be weakly positive or negative. This is because fewer antigenic sites exist on neonatal red blood cells in ABO incompatibility, resulting in less antibody attachment.
Nursing Management and Interventions
Initial Nursing Assessment
Upon admission to the nursery, the nurse should perform a comprehensive assessment of the newborn born to an Rh-negative mother:
- Review maternal history for Rh status, antibody titers, and RhoGAM administration
- Assess cord blood results (blood type, Rh factor, direct Coombs test)
- Perform thorough physical assessment focusing on:
- Skin color and presence of jaundice
- Respiratory effort and rate
- Heart rate and presence of murmurs
- Abdominal examination for hepatosplenomegaly
- Assessment for edema
- Neurological status and reflexes
- Monitor vital signs, including temperature, heart rate, respiratory rate, and blood pressure
- Assess feeding patterns and urine output
Ongoing Monitoring
Physical Assessment
- Assess for jaundice every 4-8 hours
- Monitor progression of jaundice using cephalocaudal assessment
- Assess for signs of anemia (pallor, tachycardia)
- Monitor for signs of respiratory distress
- Assess neurological status for signs of bilirubin encephalopathy
- Monitor temperature during phototherapy
Laboratory Monitoring
- Monitor serum bilirubin levels as ordered (typically every 4-12 hours depending on severity)
- Transcutaneous bilirubin measurements may be used for screening
- Monitor hemoglobin and hematocrit
- Track reticulocyte counts
- Monitor electrolytes, especially in infants receiving intensive phototherapy or exchange transfusion
- Blood glucose monitoring, particularly in infants with poor feeding
Key Nursing Interventions
| Intervention | Rationale | Nursing Considerations |
|---|---|---|
| Promote adequate hydration | Prevents dehydration and facilitates bilirubin excretion |
|
| Support and maintain phototherapy | Converts bilirubin into water-soluble isomers for excretion |
|
| Thermoregulation | Prevents hypothermia or hyperthermia during phototherapy |
|
| Support breastfeeding | Breast milk helps promote intestinal motility and bilirubin excretion |
|
| Neurological monitoring | Early detection of bilirubin encephalopathy |
|
| Prepare for and assist with exchange transfusion | Removes antibody-coated RBCs and bilirubin, replaces with donor RBCs |
|
| Administration of IVIG | Blocks Fc receptors, reducing hemolysis |
|
Critical Nursing Alert
Monitor for signs of acute bilirubin encephalopathy, which include:
- Lethargy and poor feeding
- Hypotonia followed by hypertonia
- High-pitched cry
- Backward arching of the neck and trunk (retrocollis and opisthotonus)
- Setting-sun sign (eyes fixed downward)
- Seizures
These signs represent a medical emergency requiring immediate intervention.
Treatment Options
Treatment of hemolytic disease of the newborn depends on the severity of the condition and may include:
1. Phototherapy
Phototherapy is the primary treatment for hyperbilirubinemia. It works by:
- Transforming bilirubin into water-soluble isomers (lumirubin) that can be excreted without liver conjugation
- Reducing serum bilirubin levels and preventing bilirubin encephalopathy
Types of Phototherapy
- Conventional phototherapy: Overhead lights positioned above the infant
- Intensive phototherapy: Multiple light sources from above and below (light blankets)
- Fiberoptic phototherapy: Uses a fiberoptic blanket that wraps around the infant
Nursing Considerations for Phototherapy
- Maximize skin exposure (infant wears only a diaper)
- Protect eyes with appropriate eye shields
- Shield male genitalia
- Reposition infant every 2 hours
- Monitor temperature frequently
- Remove from phototherapy during feeding when possible
- Monitor skin for rashes or burns
- Monitor hydration status (increased insensible water losses)
- Continue to encourage frequent breastfeeding
2. Intravenous Immunoglobulin (IVIG)
IVIG is used in cases of severe hemolytic disease to reduce the need for exchange transfusion.
- Mechanism of action: Blocks Fc receptors on macrophages, reducing hemolysis of antibody-coated RBCs
- Dosage: Typically 0.5-1 g/kg over 2-4 hours
- Indications: Used when bilirubin levels are rising despite intensive phototherapy or approaching exchange transfusion thresholds
- Benefits: May reduce the need for exchange transfusion by 75% in selected cases
3. Exchange Transfusion
Exchange transfusion is reserved for severe cases where bilirubin levels approach or exceed the threshold for kernicterus risk, or when severe anemia is present.
Exchange Transfusion Process
Exchange transfusion involves removing small amounts of the infant’s blood and replacing it with donor blood, typically through umbilical vessels. The process:
- Removes antibody-coated RBCs
- Removes circulating bilirubin
- Supplies donor RBCs (type O, Rh-negative)
- Corrects anemia
Indications for Exchange Transfusion
- Bilirubin levels exceeding exchange transfusion thresholds per American Academy of Pediatrics guidelines
- Signs of acute bilirubin encephalopathy
- Severe anemia at birth (hemoglobin <10 g/dL)
- Rapidly rising bilirubin levels (>0.5 mg/dL/hour) despite intensive phototherapy
Nursing Responsibilities During Exchange Transfusion
- Assist with preparation of equipment and blood products
- Continuous monitoring of vital signs
- Accurate documentation of volumes removed and infused
- Monitoring for complications (hypoglycemia, electrolyte imbalances, bleeding)
- Comfort and support for infant
- Family support and education
4. Intrauterine Transfusion (Antenatal Management)
For severely affected fetuses, intrauterine transfusions may be performed before birth:
- Performed when MCA Doppler studies indicate severe fetal anemia
- Involves direct transfusion of RBCs into the fetal circulation (usually the umbilical vein)
- May need to be repeated every 2-4 weeks until delivery
- Improved survival rates from <10% to >90% for severe cases
- Usually managed by maternal-fetal medicine specialists
Complications and Long-Term Effects
Acute Complications
Kernicterus (Bilirubin Encephalopathy)
Kernicterus is the most serious complication of severe hyperbilirubinemia, resulting from deposition of bilirubin in brain tissue.
| Stage | Clinical Manifestations |
|---|---|
| Early Stage | Lethargy, hypotonia, poor feeding, weak sucking reflex, high-pitched cry |
| Intermediate Stage | Moderate stupor, irritability, hypertonia, opisthotonos (backward arching of head and trunk), fever |
| Advanced Stage | Pronounced retrocollis and opisthotonus, setting-sun sign, seizures, shrill cry, apnea, coma |
Long-Term Sequelae of Kernicterus
If a newborn develops kernicterus, potential long-term effects include:
- Cerebral palsy: Particularly choreoathetoid type with involuntary movements
- Sensorineural hearing loss: Ranging from mild to profound
- Visual and gaze abnormalities: Upward gaze paralysis, strabismus
- Dental enamel dysplasia: Affecting tooth development
- Intellectual disabilities: Ranging from mild to severe
- Speech and language disorders: Auditory processing issues, dysarthria
- Learning disabilities: Affecting academic performance
Historical Perspective
Before the advent of RhoGAM and modern phototherapy techniques, kernicterus was a significant cause of childhood disability. Today, with proper prenatal screening and management, kernicterus is considered a “never event” in most developed healthcare systems, as it is largely preventable with appropriate care.
Other Complications of Hemolytic Disease
Hydrops Fetalis Complications
- Pulmonary hypoplasia
- Persistent pulmonary hypertension of the newborn (PPHN)
- Cardiac failure
- Pleural effusions
- Increased risk of intrauterine fetal demise
- Need for extensive respiratory support after birth
Other Potential Complications
- Thrombocytopenia
- Late anemia (beyond 2-3 weeks of life)
- Cholestasis
- Hypoglycemia
- Electrolyte imbalances
- Nutritional deficiencies
Long-Term Follow-Up
Infants who have had moderate to severe hemolytic disease should receive close follow-up care, including:
- Regular neurological examinations
- Hearing screenings
- Vision assessments
- Developmental assessments
- Monitoring for late anemia (may need supplemental iron)
- Early intervention services if developmental concerns arise
Nursing Care Plans
The following nursing care plans address the primary concerns for infants born to Rh-negative mothers with hemolytic disease:
Nursing Care Plan 1: Risk for Injury related to hyperbilirubinemia
Assessment Data
- Elevated bilirubin levels
- Jaundice
- Positive direct Coombs test
- History of Rh incompatibility
Expected Outcomes
- Infant will maintain bilirubin levels within safe range
- Infant will show no signs of bilirubin encephalopathy
- Infant will maintain adequate hydration status
Nursing Interventions
| Intervention | Rationale |
|---|---|
| Monitor bilirubin levels as ordered | Provides data on effectiveness of therapy and need for escalation of treatment |
| Assess skin color and extent of jaundice every 4 hours | Allows for visual assessment of jaundice progression |
| Implement phototherapy as ordered | Promotes conversion of bilirubin to water-soluble form for excretion |
| Ensure proper eye protection during phototherapy | Prevents retinal damage from phototherapy lights |
| Monitor neurological status every 4 hours | Early detection of bilirubin encephalopathy |
| Encourage frequent feedings (8-12 times/day) | Promotes hydration and GI motility, enhancing bilirubin excretion |
| Document stool and urine output | Monitors excretion of bilirubin and hydration status |
Evaluation
- Bilirubin levels are trending downward
- Infant shows no signs of neurological impairment
- Hydration status is adequate as evidenced by moist mucous membranes and adequate urine output
Nursing Care Plan 2: Ineffective Tissue Perfusion related to anemia
Assessment Data
- Decreased hemoglobin and hematocrit
- Pallor
- Tachycardia
- Tachypnea
- Lethargy
Expected Outcomes
- Infant will maintain adequate tissue perfusion
- Vital signs will remain within normal limits
- Hemoglobin levels will improve or stabilize
Nursing Interventions
| Intervention | Rationale |
|---|---|
| Monitor vital signs every 2-4 hours or as indicated | Detects early signs of cardiorespiratory compromise |
| Monitor oxygen saturation continuously | Assesses adequacy of oxygenation |
| Position infant for optimal respiratory effort | Promotes lung expansion and oxygen delivery |
| Monitor hemoglobin and hematocrit as ordered | Evaluates severity of anemia and need for transfusion |
| Assist with transfusion or exchange transfusion as needed | Improves oxygen-carrying capacity and removes antibody-coated RBCs |
| Minimize handling and stress | Reduces oxygen consumption and metabolic demands |
| Administer supplemental oxygen as needed | Improves tissue oxygenation |
Evaluation
- Heart rate and respiratory rate within normal limits
- Oxygen saturation >95% on room air
- Skin color improved, decreased pallor
- Hemoglobin and hematocrit stabilized or improved
Nursing Care Plan 3: Risk for Imbalanced Body Temperature related to phototherapy
Assessment Data
- Infant receiving phototherapy
- Minimal clothing (diaper only)
- Increased insensible water loss
Expected Outcomes
- Infant will maintain normal body temperature (36.5-37.5°C)
- Infant will show no signs of hypo/hyperthermia
Nursing Interventions
| Intervention | Rationale |
|---|---|
| Monitor axillary temperature every 2-4 hours | Detects temperature fluctuations early |
| Adjust environmental temperature as needed | Compensates for heat generated by phototherapy lights |
| Monitor for signs of hypothermia or hyperthermia | Early detection allows for prompt intervention |
| Ensure servo-controlled incubator/warmer if used | Provides continuous temperature regulation |
| Monitor for excessive sweating or cold extremities | Physical indicators of temperature dysregulation |
| Ensure adequate hydration | Compensates for increased insensible water loss |
Evaluation
- Infant maintains temperature between 36.5-37.5°C
- No signs of cold stress or overheating
- Adequate hydration maintained
Prevention Strategies
Prevention of Rh incompatibility and its complications involves several key strategies:
1. Maternal Screening and Monitoring
- All pregnant women should have blood typing and antibody screening at first prenatal visit
- Rh-negative women should have repeat antibody screening at 28 weeks gestation
- If antibodies are detected, regular monitoring of titers is essential
- When titers reach critical levels, referral to maternal-fetal medicine is indicated
2. RhoGAM Administration Protocol
Standard RhoGAM Protocol
- Routine antenatal prophylaxis at 28 weeks gestation (300 mcg)
- Postnatal administration within 72 hours of delivery if infant is Rh-positive (300 mcg)
- After potentially sensitizing events:
- Miscarriage or abortion: 50 mcg if <12 weeks; 300 mcg if >12 weeks
- Ectopic pregnancy: 50 mcg
- Amniocentesis, CVS: 300 mcg
- Antepartum hemorrhage: 300 mcg
- External cephalic version: 300 mcg
3. Fetal Monitoring and Intervention
For Rh-sensitized pregnancies:
- Regular monitoring of maternal antibody titers
- Middle cerebral artery (MCA) Doppler assessments starting at 18-20 weeks
- Regular ultrasound examinations to detect early signs of hydrops
- Intrauterine transfusion when indicated by fetal anemia
- Consideration of early delivery when fetal lung maturity is achieved and risk of intrauterine complications is high
4. Neonatal Screening and Early Management
- Cord blood testing for blood type, Rh factor, direct Coombs test, hemoglobin, and bilirubin
- Early initiation of feeding to promote intestinal motility and bilirubin excretion
- Close monitoring of jaundice and bilirubin levels
- Early intervention with phototherapy when indicated
- Consideration of IVIG for severe hemolytic disease
5. Patient Education
Education for Rh-negative women should include:
Preconception and Antenatal Education
- Understanding of Rh factor and its implications
- Importance of early and regular prenatal care
- Need for RhoGAM administration
- Recognition of potentially sensitizing events
- Importance of reporting bleeding or trauma during pregnancy
Postnatal Education
- Recognition of jaundice and when to seek medical attention
- Importance of frequent feeding
- Follow-up appointments for bilirubin checking
- Long-term implications for future pregnancies
- Need for medical alert identification indicating Rh sensitization status
Global Perspective on Prevention
While RhoGAM has dramatically reduced the incidence of Rh sensitization in developed countries, access remains limited in many parts of the world. International health organizations continue to work toward making RhoGAM more widely available globally. In resource-limited settings, early identification of at-risk pregnancies and referral to higher levels of care remains a critical strategy.
Mnemonics and Memory Tools
The following mnemonics will help nursing students remember key concepts related to Rh incompatibility:
R.H.O.G.A.M
Remember when to administer RhoGAM to Rh-negative mothers:
J.A.U.N.D.I.C.E
Assessment priorities for a jaundiced newborn:
K.E.R.N.I.C.T.E.R.U.S
Warning signs of bilirubin encephalopathy:
Summary
Caring for babies born to Rh-negative mothers requires a comprehensive understanding of the pathophysiology of Rh incompatibility, as well as skills in assessment, prevention, and management of hemolytic disease of the newborn.
Key Concepts
- Rh incompatibility occurs when an Rh-negative mother carries an Rh-positive fetus
- Maternal sensitization leads to production of antibodies that cross the placenta
- These antibodies attack fetal RBCs, causing hemolysis, anemia, and hyperbilirubinemia
- Prevention with RhoGAM is highly effective when properly administered
- Early detection and prompt treatment are essential to prevent complications
- Kernicterus, the most serious complication, is largely preventable with modern care
Core Nursing Responsibilities
- Identifying at-risk infants based on maternal history
- Conducting thorough assessments for jaundice and signs of anemia
- Monitoring and interpreting laboratory values
- Implementing and managing phototherapy
- Assisting with advanced interventions like IVIG and exchange transfusion
- Providing education to parents about jaundice monitoring and follow-up
- Supporting breastfeeding and adequate hydration
The dramatic reduction in severe hemolytic disease of the newborn and kernicterus over the past decades represents one of the greatest success stories in preventive medicine. Through proper screening, prophylaxis, monitoring, and treatment, this once-common and devastating condition has become rare in countries with developed healthcare systems.
However, continued vigilance is necessary, as failures in prevention or treatment can still lead to severe outcomes. Nurses play a crucial role in this process, from educating Rh-negative women about the importance of RhoGAM to providing expert care to affected newborns.
By understanding and applying the principles outlined in these notes, nursing students will be well-equipped to provide optimal care for babies born to Rh-negative mothers and help ensure the best possible outcomes for these vulnerable infants.
References
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. (2004). Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics, 114(1), 297-316. https://publications.aap.org/pediatrics
- ACOG Committee Opinion No. 757: Screening for Perinatal Depression. (2018). Obstetrics and Gynecology, 132(5), e208-e212. https://www.acog.org/clinical
- Delaney, M., & Matthews, D. C. (2015). Hemolytic disease of the fetus and newborn: managing the mother, fetus, and newborn. Hematology, 2015(1), 146-151. https://www.ncbi.nlm.nih.gov/books/NBK557423
- Hendrickson, J. E., & Delaney, M. (2016). Hemolytic disease of the fetus and newborn: modern practice and future investigations. Transfusion Medicine Reviews, 30(4), 159-164. https://www.sciencedirect.com
- Bhutani, V. K., & Wong, R. J. (2013). Bilirubin neurotoxicity in preterm infants: risk and prevention. Journal of Clinical Neonatology, 2(2), 61-69. https://www.jcnonweb.com
- Ree, I. M., Smits-Wintjens, V. E., van der Bom, J. G., van Klink, J. M., Oepkes, D., & Lopriore, E. (2017). Neonatal management and outcome in alloimmune hemolytic disease. Expert Review of Hematology, 10(7), 607-616. https://www.tandfonline.com
- de Haas, M., Thurik, F. F., Koelewijn, J. M., & van der Schoot, C. E. (2015). Haemolytic disease of the fetus and newborn. Vox Sanguinis, 109(2), 99-113. https://onlinelibrary.wiley.com
- Watchko, J. F., & Tiribelli, C. (2013). Bilirubin-induced neurologic damage—mechanisms and management approaches. New England Journal of Medicine, 369(21), 2021-2030. https://www.nejm.org
- Kaplan, M., Bromiker, R., & Hammerman, C. (2014). Hyperbilirubinemia, hemolysis, and increased bilirubin neurotoxicity. Seminars in Perinatology, 38(7), 429-437. https://www.sciencedirect.com
- Moise Jr, K. J. (2008). Management of rhesus alloimmunization in pregnancy. Obstetrics & Gynecology, 112(1), 164-176. https://journals.lww.com/greenjournal
- American College of Obstetricians and Gynecologists. (2018). ACOG Practice Bulletin No. 192: Management of Alloimmunization During Pregnancy. Obstetrics and Gynecology, 131(3), e82-e90. https://www.acog.org/clinical
- Nurseslabs. (2024). Hyperbilirubinemia (Jaundice) Nursing Care Plans. https://nurseslabs.com/hyperbilirubinemia-nursing-care-plans
