Introduction to Congenital Heart Disease
Congenital heart diseases (CHDs) are structural abnormalities of the heart that are present at birth. They affect approximately 8-10 per 1,000 live births, making them the most common type of birth defect. CHDs can range from simple defects with no symptoms to complex defects with severe, life-threatening symptoms.
The development of the heart occurs during the first 8 weeks of fetal life. During this critical period, any disruption in normal heart formation can result in congenital heart defects. These defects can involve the heart chambers, valves, or the blood vessels that carry blood to and from the heart.
Clinical Pearl:
Remember that CHDs are often multifactorial in origin, involving both genetic and environmental factors. Maternal factors that increase the risk include diabetes, phenylketonuria, alcohol consumption, certain medications (like retinoic acid, lithium, and some anticonvulsants), and viral infections during pregnancy (especially rubella).
Classification of Congenital Heart Diseases
Congenital heart diseases are commonly classified based on their hemodynamic presentation:
| Classification | Hemodynamic Effect | Oxygen Saturation | Examples |
|---|---|---|---|
| Acyanotic | Left-to-right shunt or obstructive lesions | Normal oxygen saturation in arterial blood | ASD, VSD, PDA, Coarctation of aorta |
| Cyanotic | Right-to-left shunt | Decreased oxygen saturation in arterial blood | TOF, Transposition of great arteries, Truncus arteriosus |
Mnemonic: “VIPER” for common CHDs
- Ventricular Septal Defect (VSD)
- Interrupted Aortic Arch
- Patent Ductus Arteriosus (PDA)
- Endocardial Cushion Defect
- Right-sided lesions (TOF, pulmonary stenosis)
Normal Cardiac Anatomy and Physiology
Before discussing specific heart defects, let’s briefly review normal cardiac anatomy and blood flow:

Normal heart anatomy showing chambers, valves, and blood flow direction
Normal Blood Flow Through the Heart
- Deoxygenated blood returns from the body to the right atrium via the superior and inferior venae cavae
- Blood passes through the tricuspid valve into the right ventricle
- The right ventricle pumps blood through the pulmonary valve into the pulmonary artery to the lungs for oxygenation
- Oxygenated blood returns from the lungs via the pulmonary veins to the left atrium
- Blood passes through the mitral valve into the left ventricle
- The left ventricle pumps blood through the aortic valve into the aorta to supply the body
Fetal Circulation
Fetal circulation differs from postnatal circulation in several important ways:
- The placenta, not the lungs, provides oxygenated blood to the fetus
- The foramen ovale allows blood to bypass the right ventricle and lungs by flowing directly from the right atrium to the left atrium
- The ductus arteriosus connects the pulmonary artery to the aorta, allowing blood to bypass the non-functioning lungs
- After birth, these shunts normally close as the lungs begin to function
Acyanotic Congenital Heart Defects
Atrial Septal Defect (ASD)

Diagram showing Atrial Septal Defect (ASD) with left-to-right shunt
Pathophysiology
An atrial septal defect is an abnormal opening in the wall (septum) between the left and right atria. This creates a left-to-right shunt as blood flows from the higher-pressure left atrium to the lower-pressure right atrium.
Types of ASD:
- Secundum ASD (75%): Defect in the middle portion of the atrial septum (fossa ovalis)
- Primum ASD (15-20%): Defect in the lower portion of the atrial septum near the AV valves
- Sinus venosus ASD (5-10%): Defect near the entrance of the superior vena cava
- Coronary sinus ASD (<1%): Rare defect involving the coronary sinus
Hemodynamic Effects:
In an ASD, there is a left-to-right shunt at the atrial level resulting in:
- Volume overload of the right atrium
- Volume overload of the right ventricle
- Increased pulmonary blood flow
- Left atrial volume overload (from increased pulmonary venous return)
Over time, if left untreated, pulmonary hypertension may develop, potentially leading to Eisenmenger syndrome where the shunt reverses to right-to-left.
Clinical Manifestations
Many patients with ASDs are asymptomatic, especially in childhood. Symptoms may develop with age as the right heart chambers dilate and pulmonary vascular resistance increases.
| Signs/Symptoms | Description |
|---|---|
| Heart Murmur | Fixed splitting of S₂ (hallmark finding); systolic ejection murmur at the upper left sternal border (due to increased flow across the pulmonary valve) |
| Exercise Intolerance | Fatigue and dyspnea with exertion (more common in adults) |
| Palpitations | Due to atrial arrhythmias (atrial fibrillation, atrial flutter) |
| Recurrent Respiratory Infections | Due to increased pulmonary blood flow |
| Growth Failure | In children with large defects |
| Late Manifestations | Right heart failure, peripheral edema, atrial arrhythmias, paradoxical embolism |
Clinical Pearl:
Fixed splitting of the second heart sound (S₂) that doesn’t change with respiration is the classic auscultatory finding in ASD. This occurs because the increased right ventricular volume delays pulmonic valve closure regardless of the respiratory phase.
Diagnostic Evaluation
- Echocardiography: Primary diagnostic tool – shows the defect and assesses its size, location, direction of shunt, and associated anomalies
- Electrocardiogram (ECG): Right axis deviation, incomplete right bundle branch block, prolonged PR interval
- Chest X-ray: Cardiomegaly, enlarged right atrium and ventricle, increased pulmonary vascular markings
- Cardiac Catheterization: Rarely needed but can confirm the diagnosis, quantify the shunt, and measure pulmonary vascular resistance
- Transesophageal Echocardiography (TEE): May be needed for better visualization of certain types of ASD (e.g., sinus venosus)
Treatment and Management
The management approach depends on the size of the defect, presence of symptoms, and age of the patient.
Small ASDs:
- Many small defects (< 5mm) close spontaneously during the first few years of life
- Regular monitoring with echocardiography
- No activity restrictions
Moderate to Large ASDs:
- Transcatheter Device Closure:
- Preferred for secundum ASDs with adequate rims
- Less invasive than surgery
- Shorter hospital stay
- Surgical Repair:
- Indicated for large defects, primum ASDs, sinus venosus ASDs
- Direct suture closure or patch repair
- Usually performed between 2-5 years of age
Timing of Intervention:
Closure is typically recommended for:
- All symptomatic patients
- Asymptomatic patients with significant shunts (Qp:Qs > 1.5:1)
- Defects with right heart chamber enlargement
- Prior to school age in children with hemodynamically significant defects
Important Note:
ASD closure is contraindicated in patients with irreversible pulmonary hypertension (Eisenmenger syndrome) as it may worsen right heart failure.
Nursing Considerations
Assessment:
- Auscultate for characteristic fixed splitting of S₂ and systolic ejection murmur
- Monitor for signs of heart failure (tachycardia, tachypnea, fatigue, poor feeding in infants)
- Assess growth and development in children
- Evaluate exercise tolerance and activity level
Preoperative Care:
- Provide age-appropriate education about the procedure
- Prepare family for what to expect post-procedure
- Ensure baseline vital signs are documented
- Review medication history and allergies
Postoperative Care:
- Monitor vital signs and hemodynamic parameters
- Assess for cardiac arrhythmias
- Monitor for complications including bleeding, infection, pericardial effusion
- Provide pain management
- For transcatheter closure, observe access site for bleeding or hematoma
- Gradual resumption of activities
Patient Education:
- Importance of follow-up appointments
- Activity restrictions (typically minimal after recovery)
- Wound care instructions after surgical repair
- Signs and symptoms requiring medical attention
- Endocarditis prophylaxis recommendations (typically only needed for 6 months after device closure)
Ventricular Septal Defect (VSD)
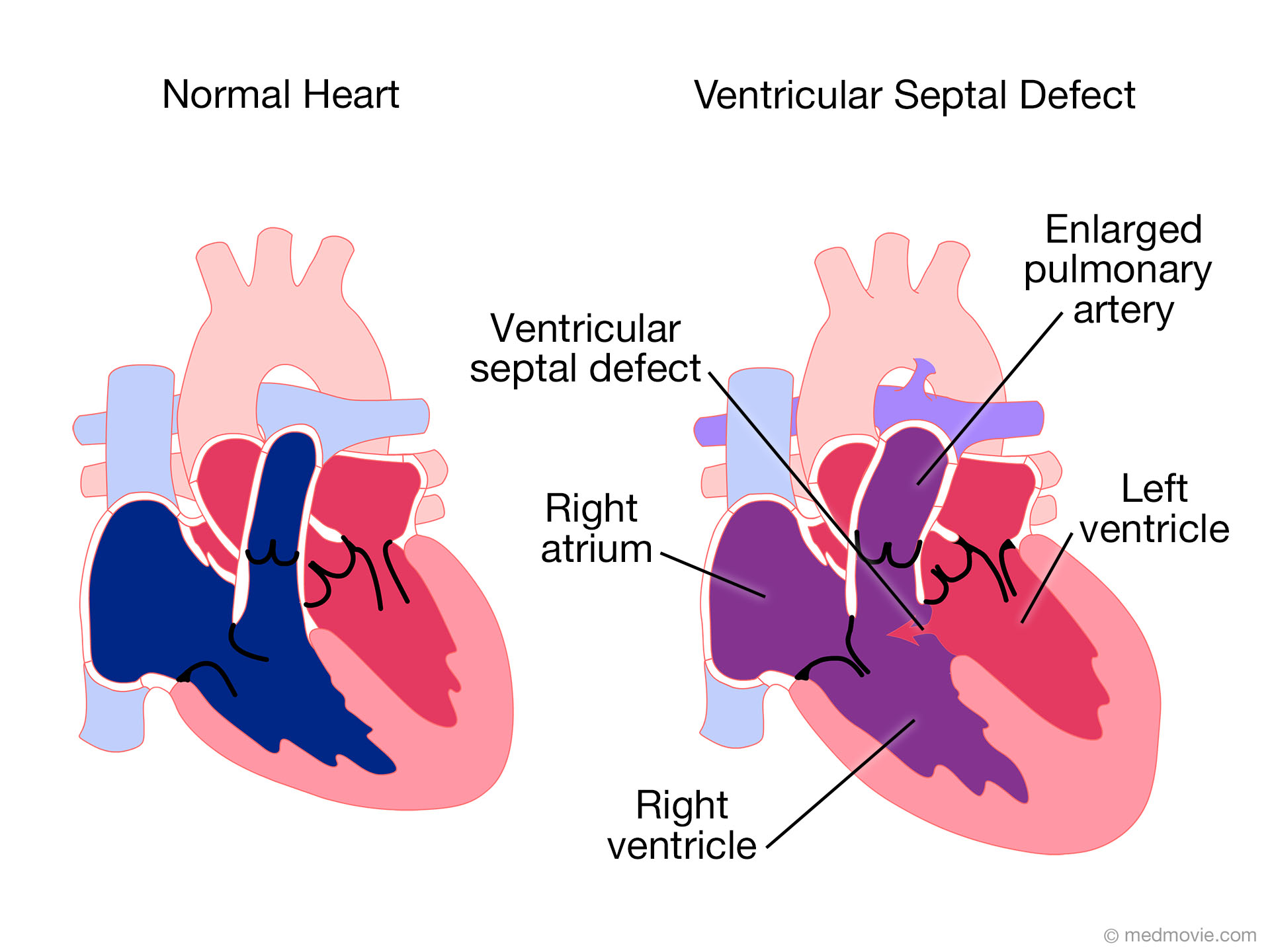
Diagram showing Ventricular Septal Defect with blood flow patterns
Pathophysiology
A ventricular septal defect is an abnormal opening in the wall (septum) between the right and left ventricles. This creates a left-to-right shunt as blood flows from the higher-pressure left ventricle to the lower-pressure right ventricle.
Types of VSD:
- Perimembranous VSD (80%): Located in the membranous septum, near the tricuspid valve
- Muscular VSD (15-20%): Located in the muscular portion of the septum; may be multiple (“Swiss cheese” defects)
- Inlet/AV Canal VSD (5%): Located in the inlet portion of the septum
- Supracristal/Outlet VSD (5%): Located just below the pulmonary and aortic valves
Hemodynamic Effects:
The hemodynamic significance depends on the size of the defect and pulmonary vascular resistance:
- Small VSD: Minimal left-to-right shunt, minimal hemodynamic effects
- Moderate VSD: Moderate left-to-right shunt, left atrial and ventricular volume overload
- Large VSD: Significant left-to-right shunt, leading to pulmonary overcirculation, left heart volume overload, and eventually pulmonary hypertension
Over time, untreated large VSDs can lead to pulmonary vascular disease and Eisenmenger syndrome, where the shunt reverses to right-to-left.
Clinical Manifestations
The clinical presentation varies based on the size of the defect and the amount of left-to-right shunting.
| VSD Size | Hemodynamics | Clinical Manifestations |
|---|---|---|
| Small (<25% of aortic valve diameter) |
Small left-to-right shunt Normal pulmonary pressure |
|
| Moderate (25-75% of aortic valve diameter) |
Moderate left-to-right shunt Mildly elevated pulmonary pressure |
|
| Large (>75% of aortic valve diameter) |
Large left-to-right shunt Pulmonary hypertension |
|
Clinical Pearl:
The classic finding in VSD is a harsh, holosystolic murmur best heard at the left lower sternal border. As pulmonary hypertension develops in patients with large VSDs, the murmur may actually decrease in intensity as the pressure gradient between ventricles decreases.
Diagnostic Evaluation
- Echocardiography: Primary diagnostic tool – identifies the defect location, size, and hemodynamic impact
- Electrocardiogram (ECG): May show left ventricular hypertrophy, biventricular hypertrophy, or be normal with small defects
- Chest X-ray: Increased pulmonary vascular markings, cardiomegaly in moderate to large defects
- Cardiac Catheterization: Used to measure shunt magnitude, pulmonary vascular resistance, and responsiveness to vasodilators in patients with suspected pulmonary hypertension
Treatment and Management
Small VSDs:
- Many close spontaneously (30-50%, especially muscular VSDs)
- Regular monitoring with echocardiography
- Endocarditis prophylaxis as per current guidelines
Moderate to Large VSDs:
- Medical Management:
- Diuretics to manage heart failure symptoms
- ACE inhibitors to reduce afterload
- Nutritional support for failure to thrive
- Surgical Repair:
- Indicated for large defects, symptoms despite medical management, failure to thrive
- Typically performed between 3-6 months of age for symptomatic infants
- Direct suture closure or patch repair
- Transcatheter Closure:
- Option for some muscular VSDs and select perimembranous VSDs
- Less invasive alternative to surgery
Timing of Intervention:
Intervention is typically recommended for:
- Infants with heart failure symptoms despite medical management
- Children with large VSDs (Qp:Qs > 2:1)
- Evidence of pulmonary hypertension
- Associated aortic valve prolapse or insufficiency
Important Note:
VSD closure is contraindicated in patients with Eisenmenger syndrome (irreversible pulmonary hypertension with right-to-left shunting) as it may worsen right heart failure.
Nursing Considerations
Assessment:
- Auscultate for characteristic holosystolic murmur
- Monitor for signs of heart failure (tachycardia, tachypnea, hepatomegaly, poor feeding)
- Assess weight gain and growth parameters
- Evaluate respiratory status for increased work of breathing
- Monitor for recurrent respiratory infections
Nursing Diagnoses:
- Decreased Cardiac Output related to heart failure
- Ineffective Breathing Pattern related to pulmonary congestion
- Imbalanced Nutrition: Less Than Body Requirements related to increased metabolic demands and poor feeding
- Activity Intolerance related to heart failure
- Risk for Infection related to pulmonary congestion
Nursing Interventions:
- For symptomatic infants:
- Administer medications as prescribed (diuretics, ACE inhibitors)
- Position semi-upright to decrease work of breathing
- Provide small, frequent feedings to conserve energy
- Monitor daily weights and strict intake and output
- Provide calorie-dense formula if needed
- Preoperative care:
- Prepare family for surgery through age-appropriate education
- Optimize nutritional status
- Prevent respiratory infections
- Postoperative care:
- Monitor hemodynamic parameters closely
- Assess for post-operative complications (bleeding, arrhythmias, infection)
- Manage pain effectively
- Progressive activity as tolerated
- Monitor for residual VSDs
Patient/Family Education:
- Recognition of heart failure symptoms
- Medication administration and side effects
- Feeding techniques to minimize energy expenditure
- Importance of regular follow-up care
- Signs and symptoms requiring immediate medical attention
- Endocarditis prevention guidelines
Patent Ductus Arteriosus (PDA)
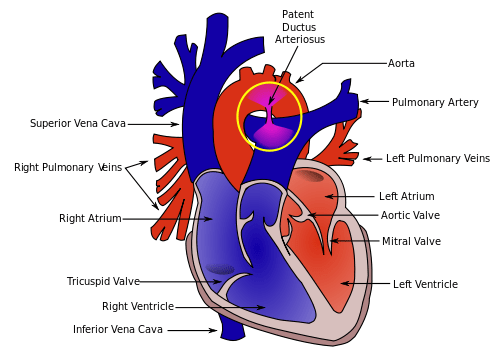
Diagram showing Patent Ductus Arteriosus connecting the aorta and pulmonary artery
Pathophysiology
The ductus arteriosus is a fetal blood vessel that connects the pulmonary artery to the descending aorta, allowing blood to bypass the non-functioning lungs during fetal life. Normally, it closes functionally within 24-48 hours after birth and anatomically within 2-3 weeks.
In patent ductus arteriosus (PDA), this vessel remains open after birth. This persistence creates a left-to-right shunt as blood flows from the higher pressure aorta to the lower pressure pulmonary artery.
Hemodynamic Effects:
The hemodynamic significance depends on the size of the PDA:
- Small PDA: Minimal left-to-right shunt with little hemodynamic effect
- Moderate PDA: Moderate left-to-right shunt leading to increased pulmonary blood flow and left heart volume overload
- Large PDA: Significant left-to-right shunt causing pulmonary overcirculation, left ventricular volume overload, and eventually pulmonary hypertension
Unlike ASDs and VSDs where shunting occurs only during specific parts of the cardiac cycle, PDAs cause continuous shunting throughout both systole and diastole since aortic pressure exceeds pulmonary artery pressure throughout the cardiac cycle.
Clinical Manifestations
The clinical presentation varies based on the size of the PDA and the patient’s age:
| Patient Group | Clinical Presentation |
|---|---|
| Preterm Infants |
|
| Term Infants/Children |
|
| Adults |
|
Clinical Pearl:
The classic “machinery” murmur of PDA is continuous throughout systole and diastole, with a peak in intensity late in systole. It is best heard in the left infraclavicular area or upper left sternal border. The name “machinery” refers to its resemblance to the sound of machinery running.
Diagnostic Evaluation
- Echocardiography: Primary diagnostic tool – shows the PDA and helps assess its size, direction of shunt, and associated cardiac abnormalities
- Electrocardiogram (ECG): May show left ventricular hypertrophy in moderate to large PDAs; usually normal with small PDAs
- Chest X-ray: May show increased pulmonary vascular markings, cardiomegaly, enlarged left atrium and ventricle in moderate to large PDAs
- Cardiac Catheterization: Rarely needed for diagnosis but may be performed as part of a therapeutic intervention
Treatment and Management
Preterm Infants:
- Medical Management:
- NSAIDs (Indomethacin, Ibuprofen): Inhibit prostaglandin synthesis to promote ductal closure
- Acetaminophen: Alternative for patients with contraindications to NSAIDs
- Fluid restriction, diuretics, and ventilatory support as needed
- Surgical Ligation:
- For PDAs that fail to close with medical management
- For infants with contraindications to medical therapy
- For symptomatic PDAs causing hemodynamic compromise
Term Infants, Children, and Adults:
- Small, Asymptomatic PDA:
- Closure generally recommended to eliminate risk of endocarditis
- Some clinicians may opt for observation of very small PDAs
- Symptomatic or Moderate/Large PDA:
- Closure is indicated
- Transcatheter Closure:
- Preferred method for most patients beyond the neonatal period
- Uses coils or occluder devices
- Less invasive than surgery with shorter recovery time
- Surgical Closure:
- For very large PDAs
- For PDAs with unfavorable anatomy for device closure
- When transcatheter approach fails or is unavailable
Important Note:
PDA closure is contraindicated in patients with Eisenmenger syndrome (irreversible pulmonary hypertension with right-to-left shunting) as it may worsen right heart failure. It is also contraindicated in certain congenital heart defects where the PDA provides essential pulmonary blood flow.
Nursing Considerations
Assessment:
- Auscultate for the characteristic continuous “machinery” murmur
- Assess peripheral pulses (bounding pulses with wide pulse pressure)
- Monitor for signs of heart failure (tachycardia, tachypnea, respiratory distress)
- In neonates, monitor for worsening respiratory status and difficulty weaning from ventilatory support
- Assess growth parameters in infants and children
Nursing Care for Medical Management (Preterm Infants):
- Administer NSAIDs as prescribed, monitoring for side effects (oliguria, elevated creatinine, bleeding)
- Monitor fluid balance carefully
- Provide respiratory support as needed
- Monitor for signs of necrotizing enterocolitis (abdominal distension, bloody stools) as a potential side effect of indomethacin
Nursing Care for Transcatheter Closure:
- Pre-procedure:
- Explain procedure to patient/family
- Ensure NPO status as per protocol
- Administer pre-medications as ordered
- Post-procedure:
- Monitor vital signs and hemodynamic status
- Assess peripheral pulses and color of extremities
- Monitor access site for bleeding or hematoma
- Keep patient on bed rest as prescribed
- Auscultate heart sounds (murmur should diminish or disappear)
- Monitor for complications: device embolization, hemolysis, vascular injury
Nursing Care for Surgical Closure:
- Preoperative:
- Prepare patient/family for surgery
- Ensure NPO status
- Complete preoperative checklist
- Postoperative:
- Monitor vital signs, hemodynamic parameters, respiratory status
- Assess incision site for signs of infection
- Manage pain effectively
- Monitor for complications: bleeding, pneumothorax, chylothorax, recurrent laryngeal nerve injury
- Progress activity as tolerated
Patient/Family Education:
- Explanation of the condition and treatment options
- Signs and symptoms requiring medical attention
- Activity restrictions after procedure
- Follow-up care requirements
- Endocarditis prophylaxis recommendations
Mnemonic for PDA Assessment: “PATENT”
- P: Pulses (bounding with wide pulse pressure)
- A: Auscultation (continuous machinery murmur)
- T: Tachypnea/Tachycardia (signs of heart failure)
- E: Exercise intolerance (in older children)
- N: Nutritional status (poor feeding, failure to thrive)
- T: Thermodynamics (hyperdynamic circulation)
Cyanotic Congenital Heart Defect
Tetralogy of Fallot (TOF)
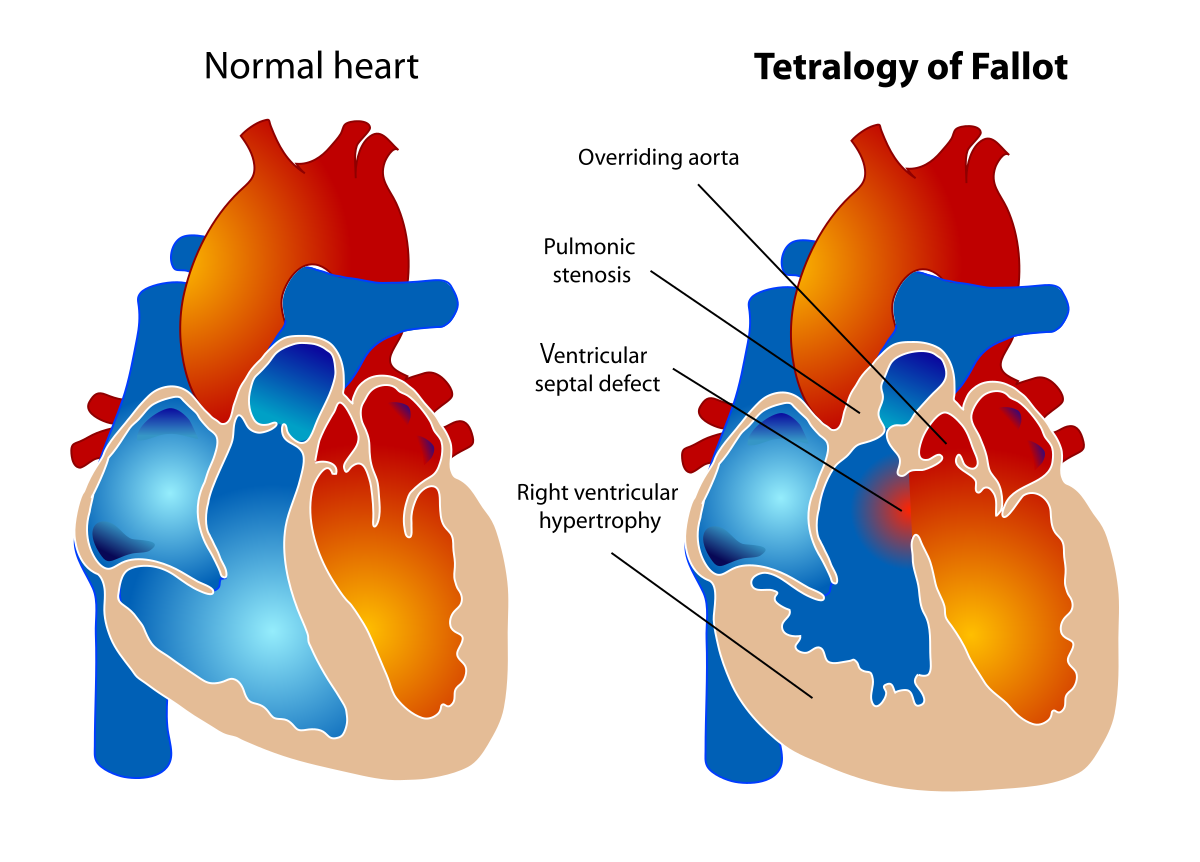
Diagram showing the four components of Tetralogy of Fallot
Pathophysiology
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, accounting for approximately 5-10% of all congenital heart diseases. It consists of four anatomical abnormalities:
The Four Components of TOF:
| Component | Description |
|---|---|
| 1. Ventricular Septal Defect (VSD) | A large hole between the left and right ventricles, usually in the perimembranous septum |
| 2. Pulmonary Stenosis | Narrowing at or below the pulmonary valve causing obstruction to blood flow from the right ventricle to the pulmonary artery |
| 3. Overriding Aorta | The aorta is positioned directly over the VSD, receiving blood from both the right and left ventricles |
| 4. Right Ventricular Hypertrophy | Thickening of the right ventricular muscle in response to the pulmonary stenosis |
Hemodynamic Effects:
The primary hemodynamic problem in TOF is reduced pulmonary blood flow due to pulmonary stenosis, which results in:
- Right-to-left shunting through the VSD causing deoxygenated blood to enter the systemic circulation
- The degree of cyanosis is directly related to the severity of the pulmonary stenosis
- Blood preferentially flows from the right ventricle to the aorta (path of least resistance) when pulmonary stenosis is severe
- Reduced pulmonary blood flow leads to chronic hypoxemia
Mnemonic: “PROVE” for Tetralogy of Fallot components
- P: Pulmonary stenosis
- R: Right ventricular hypertrophy
- O: Overriding aorta
- V: Ventricular septal defect
- E: Everything stems from anterior displacement of the infundibular septum
Clinical Manifestations
The clinical presentation varies based on the severity of pulmonary stenosis and the degree of right-to-left shunting.
Infants and Children:
- Cyanosis: May be present at birth or develop gradually over the first year of life; may be mild to severe depending on the degree of pulmonary stenosis
- Hypercyanotic (“Tet”) Spells: Episodes of sudden, severe cyanosis often triggered by crying, feeding, or defecation
- Clubbing: Develops in fingers and toes due to chronic hypoxemia
- Growth Retardation: Poor weight gain due to chronic hypoxemia
- Squatting Position: Older children may spontaneously squat to improve symptoms
- Exercise Intolerance: Limited ability to participate in physical activities
Physical Examination Findings:
- Cyanosis: Central cyanosis affecting mucous membranes, lips, and tongue
- Heart Murmur: Systolic ejection murmur at the left upper sternal border due to pulmonary stenosis
- Single S2: The pulmonic component of S2 may be reduced or absent
- Right Ventricular Heave: Palpable thrill at the left sternal border
- Clubbing: Of fingers and toes in children with long-standing cyanosis
Hypercyanotic “Tet” Spells:
Characterized by sudden increasing cyanosis, irritability, hyperpnea, and decreased intensity of heart murmur. These life-threatening episodes occur due to spasm of the infundibular muscle, increasing the right-to-left shunt. These spells require immediate intervention to prevent cerebral hypoxia and death.
Diagnostic Evaluation
- Echocardiography: Primary diagnostic tool – identifies all four components of TOF, assesses the severity of pulmonary stenosis, and evaluates associated anomalies
- Electrocardiogram (ECG): Right ventricular hypertrophy, right axis deviation, right atrial enlargement
- Chest X-ray: “Boot-shaped” heart (coeur en sabot) due to right ventricular hypertrophy and a small pulmonary trunk; decreased pulmonary vascular markings
- Cardiac MRI: Provides detailed anatomy of pulmonary arteries and coronary arteries; useful for surgical planning
- Cardiac Catheterization: Less commonly used for diagnosis but may be performed to assess pulmonary artery anatomy, coronary artery anatomy, or for interventional procedures
- Pulse Oximetry: Reveals decreased oxygen saturation
- Laboratory Studies: Polycythemia (elevated hemoglobin and hematocrit) in response to chronic hypoxemia
Clinical Pearl:
The classic “boot-shaped” heart on chest X-ray is caused by upward displacement of the cardiac apex due to right ventricular hypertrophy and a concave pulmonary artery segment due to decreased pulmonary blood flow.
Treatment and Management
Initial Management:
- Prostaglandin E1 (PGE1): For severely cyanotic neonates to maintain ductal patency and improve pulmonary blood flow
- Management of Hypercyanotic Spells:
- Knee-chest position to increase systemic vascular resistance
- Oxygen administration
- Morphine to reduce infundibular spasm
- IV fluids to improve right ventricular filling
- Sodium bicarbonate for metabolic acidosis
- Beta-blockers (such as propranolol) to reduce infundibular spasm
- Phenylephrine to increase systemic vascular resistance
Definitive Treatment:
The definitive treatment for TOF is surgical repair, which may be approached in stages or as a complete repair:
- Palliative Procedures:
- Modified Blalock-Taussig Shunt: A Gore-Tex tube connecting the subclavian artery to the pulmonary artery to increase pulmonary blood flow
- Used for severely cyanotic infants too small for complete repair
- Provides temporary improvement until complete repair can be performed
- Complete Repair:
- Closure of the VSD with a patch
- Relief of right ventricular outflow tract obstruction
- Pulmonary valvotomy or valvectomy if needed
- Enlargement of pulmonary arteries if necessary
- Typically performed between 3-6 months of age, but may be performed earlier or later depending on symptoms and anatomy
Long-term Follow-up:
- Regular cardiology follow-up with echocardiography
- Monitoring for residual or recurrent defects
- Assessment for pulmonary regurgitation which often develops after repair
- Pulmonary valve replacement may be needed in adulthood
- Monitoring for arrhythmias
- Exercise restrictions based on individual assessment
- Endocarditis prophylaxis as per current guidelines
Nursing Considerations
Assessment:
- Monitor oxygen saturation levels continuously
- Assess for signs of increasing cyanosis, particularly during crying or feeding
- Monitor for signs of hypercyanotic spells
- Auscultate heart sounds and monitor for changes in murmur intensity
- Assess growth parameters
- Evaluate activity tolerance and developmental milestones
- Monitor for complications of chronic hypoxemia (polycythemia, clubbing)
Nursing Diagnoses:
- Impaired Gas Exchange related to right-to-left shunting
- Activity Intolerance related to decreased oxygen delivery to tissues
- Ineffective Tissue Perfusion (cerebral) related to hypoxemia
- Risk for Delayed Growth and Development related to chronic hypoxemia
- Anxiety (parental) related to child’s condition and upcoming surgery
Nursing Interventions for Hypercyanotic Spells:
- Place child in knee-chest position
- Administer oxygen via mask
- Provide calm environment, minimize crying
- Administer medications as prescribed (morphine, propranolol, phenylephrine)
- Monitor oxygen saturation and level of consciousness continuously
- Prepare for possible emergency interventions
- Document frequency, duration, and triggers of spells
Preoperative Care:
- Provide thorough preoperative education to family
- Optimize nutritional status
- Monitor and prevent hypercyanotic spells
- Administer prophylactic antibiotics as ordered
- Prepare family for ICU environment and postoperative appearance
Postoperative Care:
- Monitor hemodynamic stability closely
- Assess for signs of decreased cardiac output
- Monitor for surgical complications (bleeding, arrhythmias, heart block)
- Provide effective pain management
- Gradually increase activity as tolerated
- Monitor fluid balance carefully
- Assess for post-pericardiotomy syndrome (fever, chest pain, friction rub)
Patient/Family Education:
- Recognition and management of hypercyanotic spells
- Importance of adequate nutrition and hydration
- Endocarditis prophylaxis recommendations
- Wound care after surgery
- Signs and symptoms requiring medical attention
- Activity restrictions and gradual return to normal activities
- Importance of regular cardiology follow-up
Teaching for Parents: Managing Hypercyanotic Spells
Teach parents the “CALM” approach for managing hypercyanotic spells:
- C: Calm the child, provide comfort
- A: Assist into knee-chest position
- L: Low-flow oxygen if available
- M: Medical attention if spell persists or worsens
Comparison of Congenital Heart Defects
| Feature | ASD | VSD | PDA | TOF |
|---|---|---|---|---|
| Category | Acyanotic | Acyanotic | Acyanotic | Cyanotic |
| Shunt Direction | Left-to-right | Left-to-right | Left-to-right | Right-to-left |
| Primary Defect | Opening between atria | Opening between ventricles | Persistent fetal vessel | Four cardiac anomalies |
| Characteristic Murmur | Systolic ejection murmur with fixed split S₂ | Harsh holosystolic murmur | Continuous “machinery” murmur | Systolic ejection murmur |
| Typical Symptoms in Infants | Often asymptomatic | Heart failure, poor feeding | Heart failure, respiratory symptoms | Cyanosis, hypercyanotic spells |
| Spontaneous Closure | Common for small secundum ASDs | Common (30-50%) | Rare after neonatal period | Does not close spontaneously |
| Treatment Approach | Device closure or surgical repair | Medical management or surgical repair | Medical closure in premature infants; device or surgical closure in others | Surgical repair (palliative shunt or complete repair) |
| Long-term Complications | Atrial arrhythmias, pulmonary hypertension | Heart failure, pulmonary hypertension | Endarteritis, heart failure, pulmonary hypertension | Pulmonary regurgitation, arrhythmias, heart failure |
Clinical Assessment: Red Flags for Congenital Heart Disease
Signs Warranting Urgent Evaluation:
- Central cyanosis (blue discoloration of lips and tongue)
- Respiratory distress with feeding
- Failure to thrive despite adequate caloric intake
- Abnormal heart murmur with other concerning symptoms
- Tachypnea (respiratory rate > 60 breaths/min) at rest
- Diaphoresis (excessive sweating) during feeding
- Diminished femoral pulses
- Hypercyanotic spells
Mnemonic: “CARDIAC” for Nursing Assessment of CHD
- C: Color (cyanosis, pallor, mottling)
- A: Activity level and tolerance
- R: Respiratory status (rate, effort, pattern)
- D: Development and growth (weight gain, milestones)
- I: Intake and output (feeding difficulties, diaphoresis)
- A: Auscultation findings (murmurs, extra heart sounds)
- C: Clubbing and other chronic hypoxemia signs
Key Nursing Interventions for Children with CHD
| Intervention Area | Key Actions |
|---|---|
| Nutritional Support |
|
| Respiratory Management |
|
| Infection Prevention |
|
| Promoting Development |
|
| Family Support |
|
References
- Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. 9th ed. Wolters Kluwer; 2016.
- Park MK. Park’s Pediatric Cardiology for Practitioners. 7th ed. Elsevier; 2020.
- Hockenberry MJ, Wilson D. Wong’s Nursing Care of Infants and Children. 11th ed. Elsevier; 2018.
- Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. Circulation. 2012;126(9):1143-1172.
- American Heart Association. Congenital Heart Defects in Children. https://www.heart.org/en/health-topics/congenital-heart-defects
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease. Circulation. 2019;139(14):e698-e800.
- Bailliard F, Anderson RH. Tetralogy of Fallot. Orphanet J Rare Dis. 2009;4:2.
- Chowdhury D. Pathophysiology of congenital heart diseases. Ann Card Anaesth. 2007;10(1):19-26.
