Chemistry Fundamentals for Nursing Students
Master Atomic Structure & Periodic Properties
Table of Contents
Basic Concepts of Chemistry
Matter and Its States
Matter is anything that occupies space and has mass. Understanding matter is crucial for nursing practice as medications, body fluids, and biological substances exist in different states.
- Solid: Definite shape and volume (tablets, capsules)
- Liquid: Definite volume, variable shape (IV fluids, syrups)
- Gas: Variable shape and volume (oxygen, anesthetics)
Elements, Compounds & Mixtures
These classifications help nurses understand drug composition and patient physiology at the molecular level.
Memory Helper – States of Matter
“Sally Loves Giving Pills”
Solid → Liquid → Gas → Plasma (energy levels increasing)
Chemical Bonding Fundamentals
Ionic Bonds
Transfer of electrons between metals and non-metals. Common in electrolytes like NaCl.
Covalent Bonds
Sharing of electrons. Found in organic molecules and most medications.
Hydrogen Bonds
Weak attractions crucial for protein structure and DNA stability.
Structure of Atom
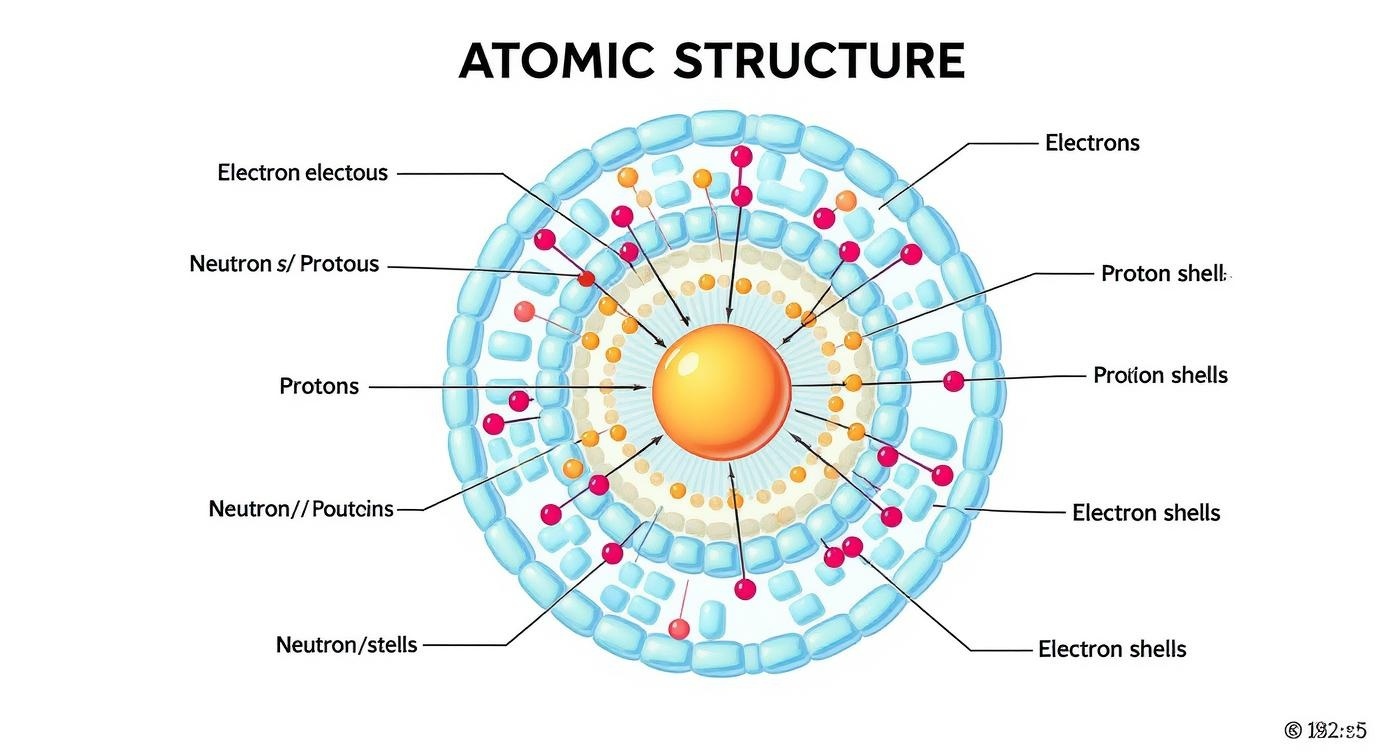
Atomic structure showing the fundamental components of matter
Subatomic Particles
Protons
- • Positive charge (+1)
- • Located in nucleus
- • Mass: ~1 atomic mass unit (amu)
- • Determines atomic number
Neutrons
- • Neutral charge (0)
- • Located in nucleus
- • Mass: ~1 amu
- • Provides atomic stability
Electrons
- • Negative charge (-1)
- • Orbit around nucleus
- • Mass: ~1/1836 amu
- • Determine chemical properties
Electron Configuration
Electrons occupy specific energy levels (shells) around the nucleus. Understanding electron configuration is crucial for predicting how elements will react, which is essential for understanding drug interactions and biological processes.
Energy Levels (Shells):
Electron Shell Visualization
Key Atomic Concepts
Atomic Number (Z)
Number of protons in nucleus
Defines the element’s identity
Atomic Mass (A)
Protons + Neutrons
Determines isotope variation
Memory Helper – Atomic Components
“Positive Protons Need Neutral Neutrons, Energetic Electrons Everywhere”
Remember: Protons (+), Neutrons (0), Electrons (-)
Classification of Elements
The periodic table organizes elements based on their atomic structure and properties. For nursing students, understanding element classification helps in comprehending drug mechanisms, electrolyte balance, and biological processes.
Simplified Periodic Table (Major Elements)
Metals
Good conductors, malleable, and tend to lose electrons.
Non-metals
Poor conductors, brittle, and tend to gain electrons.
Metalloids
Properties between metals and non-metals.
Groups and Periods
Groups (Vertical Columns)
Elements with similar chemical properties
- Group 1: Alkali metals (Na, K)
- Group 2: Alkaline earth metals (Ca, Mg)
- Group 17: Halogens (Cl, F)
- Group 18: Noble gases (He, Ar)
Periods (Horizontal Rows)
Elements with same number of electron shells
- Period 1: H, He (1 shell)
- Period 2: Li to Ne (2 shells)
- Period 3: Na to Ar (3 shells)
Memory Helper – Important Groups
“All Amazing Chemists Have Noble Goals”
Alkali → Alkaline earth → Chalcogens → Halogens → Noble gases
Periodicity in Properties
Periodic trends help predict element behavior and are crucial for understanding how different substances interact in the human body. These atomic properties directly influence drug action, toxicity, and therapeutic effects.
Major Periodic Trends
Atomic Radius
The size of an atom affects how it interacts with other atoms and molecules.
Ionization Energy
Energy required to remove an electron from an atom.
Electronegativity
Ability of an atom to attract electrons in a bond.
Metallic Character
Tendency to lose electrons and form positive ions.
| Property | Across Period (→) | Down Group (↓) | Nursing Application |
|---|---|---|---|
| Atomic Radius | Decreases | Increases | Drug-receptor fit and selectivity |
| Ionization Energy | Increases | Decreases | Electrolyte stability and reactivity |
| Electronegativity | Increases | Decreases | Molecular polarity, drug solubility |
| Metallic Character | Decreases | Increases | Ion formation, mineral supplements |
Memory Helper – Periodic Trends
“Right Increases Ion Energy” (Across periods)
“Down Drops Ion Energy” (Down groups)
Remember: Moving right increases ionization energy and electronegativity, but decreases atomic radius and metallic character
Chemistry Applications in Nursing Practice
Pharmacology Applications
Physiological Processes
Laboratory Values
Medication Administration
Clinical Scenarios: Chemistry in Action
Scenario 1: Electrolyte Imbalance
Patient with severe diarrhea showing signs of dehydration and muscle weakness.
Chemistry concept: Ionic bonds and electrolyte function
Application: Understanding Na⁺, K⁺, Cl⁻ balance for replacement therapy
Atomic relevance: Ion size affects membrane transport
Scenario 2: Oxygen Therapy
Patient with COPD requiring precise oxygen concentration monitoring.
Chemistry concept: Gas laws and molecular behavior
Application: Understanding O₂-hemoglobin binding kinetics
Atomic relevance: Electron configuration of iron in hemoglobin
Scenario 3: Drug Compatibility
Mixing multiple IV medications in same line – assessing compatibility.
Chemistry concept: Chemical reactions and molecular interactions
Application: Predicting precipitation and degradation
Atomic relevance: Electronegativity differences affecting stability
Scenario 4: Acid-Base Disorders
Patient with diabetic ketoacidosis showing metabolic acidosis.
Chemistry concept: Proton transfer and buffer systems
Application: Understanding bicarbonate buffer mechanism
Atomic relevance: Hydrogen bonding in buffer systems
Key Takeaways for Nursing Practice
- Atomic structure determines drug behavior and biological interactions
- Periodic trends help predict element toxicity and therapeutic effects
- Chemical bonding affects drug solubility and absorption
- Electronegativity influences molecular polarity and membrane permeability
- Ion size affects cellular transport and electrolyte balance
- Understanding atomic properties enhances medication safety
Global Best Practices in Chemistry Education for Healthcare
Scandinavian Model (Norway, Sweden, Denmark)
Canadian Approach
Japanese Innovation
Netherlands Excellence
Implementation Recommendations
Technology Integration
- • Virtual atomic structure models
- • Interactive periodic table apps
- • Augmented reality chemistry lab
- • Online simulation platforms
Collaborative Learning
- • Peer tutoring programs
- • Study group formations
- • Case-based discussions
- • Interprofessional education
Assessment Methods
- • Competency-based evaluation
- • Portfolio development
- • Practical applications testing
- • Continuous feedback loops
Emerging Global Trends
Educational Innovations
- AI-powered personalized learning paths
- Virtual reality atomic structure exploration
- Mobile-first chemistry learning platforms
- Gamified periodic table mastery
Assessment Evolution
- Real-time competency tracking
- Cognitive load optimization
- Adaptive testing algorithms
- Micro-credentialing systems
Quick Reference Summary
Atomic Structure
Protons, neutrons, electrons determine chemical behavior
Periodic Table
Elements organized by atomic number and properties
Periodic Trends
Predictable patterns in atomic properties
Clinical Applications
Chemistry principles guide nursing practice
