Culture and Media Preparation in Microbiology
A Comprehensive Guide for Nursing Students
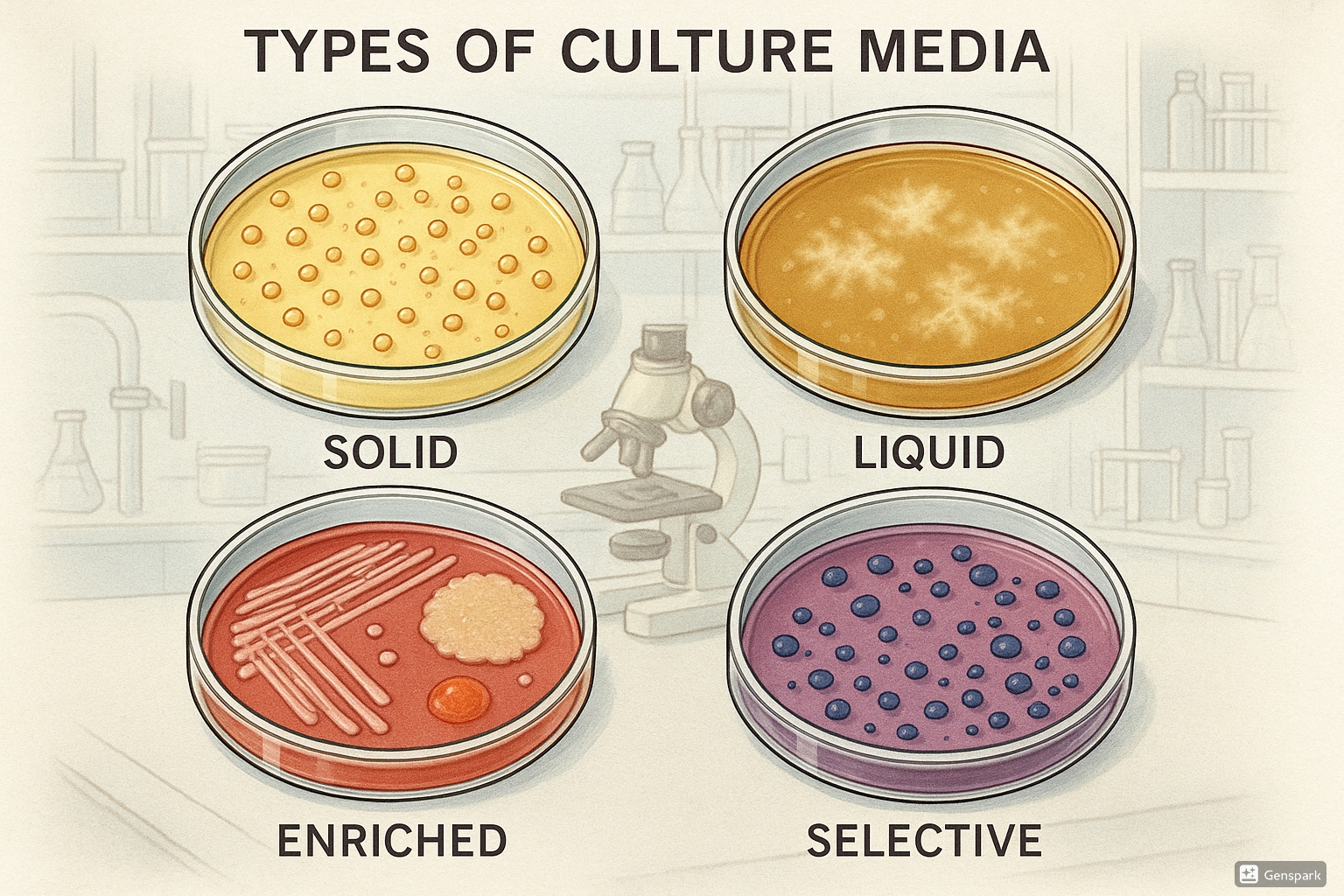
Table of Contents
- 1. Introduction to Culture Media
- 2. Basic Principles of Media Preparation
- 3. Solid Culture Media Preparation
- 4. Liquid Culture Media Preparation
- 5. Types of Culture Media
- 6. Pure Culture Techniques
- 7. Anaerobic Cultivation of Bacteria
- 8. Nursing Implications in Clinical Microbiology
- 9. Quality Control in Media Preparation
- 10. Conclusion
1. Introduction to Culture Media
Culture media are substances used in microbiology laboratories to grow and cultivate microorganisms. They provide the essential nutrients, growth factors, and environmental conditions necessary for microbial growth and reproduction. For nursing students, understanding culture media is fundamental to comprehending diagnostic procedures, infection control protocols, and antimicrobial stewardship.
Culture media serve several critical purposes in clinical microbiology:
- Isolation and identification of pathogenic microorganisms
- Antimicrobial susceptibility testing
- Study of microbial characteristics and behavior
- Production of microbial products (e.g., vaccines, antibiotics)
- Quality control in healthcare settings
Nursing Implementation
Nurses play a vital role in the proper collection, handling, and transport of clinical specimens for culture. Understanding culture media principles helps nurses in:
- Selecting appropriate collection methods for different specimen types
- Preventing contamination during specimen collection
- Ensuring proper timing of specimen collection (e.g., before antimicrobial therapy when possible)
- Interpreting culture results to guide patient care decisions
- Educating patients about the purpose and procedure of specimen collection
2. Basic Principles of Media Preparation
The preparation of culture media follows strict principles to ensure consistency, reliability, and reproducibility of results. These principles apply to both solid and liquid media formulations.
Essential Components of Culture Media
Most culture media contain the following basic components:
| Component | Function | Examples |
|---|---|---|
| Water | Solvent for nutrients; essential for metabolic reactions | Distilled or deionized water |
| Energy source | Provides carbon and energy | Glucose, sucrose, lactose |
| Nitrogen source | Required for protein synthesis | Peptones, yeast extract, meat extract |
| Vitamins and growth factors | Essential for metabolism | B-complex vitamins, amino acids |
| Minerals and salts | Maintain osmotic balance and provide trace elements | NaCl, KH₂PO₄, MgSO₄ |
| Buffering agents | Maintain optimal pH | Phosphate buffers |
| Solidifying agents (for solid media) | Provide solid surface for growth | Agar, gelatin |
Mnemonic: “WINNERS”
To remember the essential components of culture media:
- Water (base solvent)
- Indicators (pH indicators or selective agents)
- Nutrients (carbon and nitrogen sources)
- Necessary minerals (salts and trace elements)
- Essential growth factors (vitamins, amino acids)
- Regulators (pH buffers)
- Solidifying agents (agar, gelatin)
General Steps in Media Preparation
- Weighing and mixing dry ingredients according to formulation
- Dissolving components in distilled/deionized water
- Adjusting pH to the required value (usually 7.0-7.4 for most bacteria)
- Dispensing into appropriate containers
- Sterilization (most commonly by autoclaving at 121°C, 15 psi for 15-20 minutes)
- Cooling and storage under appropriate conditions
- Quality control testing before use
Note: The 3 Ps of Media Preparation
Precision in measurement, PH adjustment, and Proper sterilization are critical for successful media preparation.
3. Solid Culture Media Preparation
Solid culture media are essential for isolating pure cultures, observing colony morphology, and conducting various microbiological tests. The most common solidifying agent used is agar, a polysaccharide derived from seaweed.
Advantages of Agar as a Solidifying Agent
- Not metabolized by most microorganisms
- Melts at approximately 95°C but solidifies at 42-45°C (preventing thermal damage to heat-sensitive additives)
- Creates a firm surface that remains solid at incubation temperatures (35-37°C)
- Transparent, allowing visualization of colonies
- Does not interfere with nutrient diffusion
Preparation of Solid Media with Agar
Types of Solid Media Preparations
| Type | Preparation Method | Applications |
|---|---|---|
| Petri Dish (Plate) | Molten agar poured into flat, round dishes | Isolation of pure cultures, colony counting, antimicrobial susceptibility testing |
| Slant | Tubes filled partially and allowed to solidify at an angle | Culture maintenance, providing larger surface area |
| Deep | Tubes filled with agar and allowed to solidify vertically | Studying oxygen requirements, motility tests |
| Butt | Small amount of agar in tube, solidified vertically | Biochemical tests (e.g., TSI agar) |
Nursing Implementation
Understanding solid media preparation helps nurses in:
- Recognizing the appropriate media for different specimen types
- Interpreting laboratory reports that describe colony morphology on solid media
- Communicating effectively with laboratory personnel about culture requirements
- Educating patients about the purpose of culture tests and expected wait times for results
4. Liquid Culture Media Preparation
Liquid media, also known as broths, are essential for various microbiological procedures including enrichment of samples, antibiotic susceptibility testing, and growth of organisms for biochemical tests.
Advantages of Liquid Media
- Allows for rapid multiplication of microorganisms
- Facilitates uniform distribution of nutrients
- Supports growth of fastidious organisms
- Can be used for quantitative analysis (e.g., MPN method)
- Ideal for bulk cultivation of microorganisms
Preparation of Liquid Media
Common Liquid Media in Clinical Microbiology
| Media | Composition | Applications |
|---|---|---|
| Nutrient Broth | Peptone, beef extract, sodium chloride | General purpose growth medium |
| Brain Heart Infusion (BHI) Broth | Infusion of brain and heart tissues, peptone, glucose | Cultivation of fastidious organisms |
| Thioglycollate Broth | Casein, yeast extract, glucose, sodium thioglycollate | Growth of anaerobes, testing sterility |
| Selenite F Broth | Peptone, lactose, sodium selenite | Selective enrichment of Salmonella species |
| Blood Culture Media | Nutrient base with SPS (sodium polyanethol sulfonate) | Detection of bacteremia and fungemia |
Mnemonic: “BROTH”
For remembering key properties of liquid media:
- Bulk cultivation capability
- Rapid growth promotion
- Optimal distribution of nutrients
- Turbidity indicates growth
- Helps in enrichment procedures
Nursing Implementation
Knowledge of liquid media is important for nurses when:
- Collecting blood culture specimens (understanding the principles of blood culture media)
- Interpreting preliminary culture results (e.g., turbidity or color change in broth)
- Timing the collection of follow-up specimens based on enrichment procedures
- Understanding the rationale for multiple sample collections in cases of suspected bacteremia
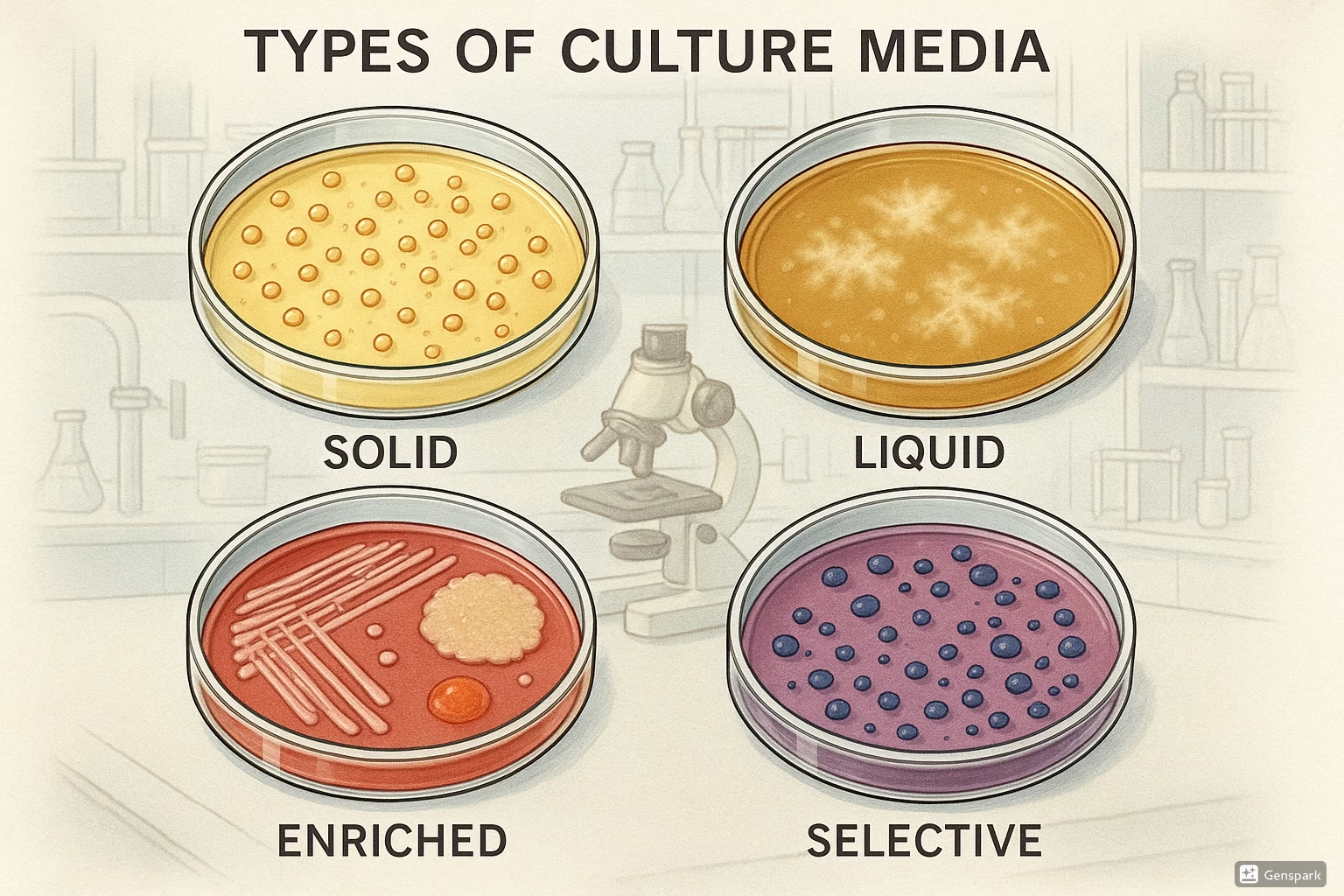
5. Types of Culture Media
Culture media can be classified based on their composition, consistency, and functional characteristics. Understanding these classifications helps in selecting the appropriate media for specific diagnostic purposes.
5.1 Semi-synthetic Media
Semi-synthetic media contain both naturally occurring ingredients and chemically defined components. They provide a balance between the nutritional complexity of natural media and the precision of synthetic formulations.
Key Characteristics:
- Contain partially processed natural ingredients (e.g., peptones, meat extracts)
- Supplemented with defined chemicals (e.g., specific sugars, salts)
- More consistent than completely natural media
- Support growth of a wide range of microorganisms
Common Examples:
- Nutrient Agar/Broth – Contains peptone (digest of animal protein) and beef extract
- MacConkey Agar – Contains peptone, lactose, bile salts, and neutral red indicator
- Tryptic Soy Agar/Broth – Contains enzymatic digests of casein and soybean meal
5.2 Synthetic Media
Synthetic (or defined) media are composed entirely of chemically defined ingredients with precisely known molecular compositions. They offer the highest level of standardization and reproducibility.
Key Characteristics:
- Exact chemical composition is known
- Highly reproducible across batches
- Allow for precise metabolic and nutritional studies
- Often less nutritionally complex than natural or semi-synthetic media
Common Examples:
- Minimal Salt Media – Contains only essential inorganic salts and a carbon source
- Chemically Defined Media (CDM) – Used for specific research purposes
- M9 Minimal Medium – Used for molecular biology applications
5.3 Enriched Media
Enriched media contain additional nutrients such as blood, serum, or specific growth factors to support the growth of nutritionally fastidious (demanding) microorganisms.
Key Characteristics:
- Supplemented with complex biological materials
- Support growth of organisms with special nutritional requirements
- Commonly used for primary isolation of clinical specimens
- May contain growth factors not available in basic media
Common Examples:
- Blood Agar – Nutrient agar supplemented with 5-10% sheep blood
- Chocolate Agar – Blood agar where red blood cells are lysed by heating
- Loeffler’s Serum Medium – Contains beef serum for Corynebacterium
Mnemonic: “FEAST”
For remembering fastidious organisms that require enriched media:
- F – Francisella
- E – Erysipelothrix
- A – Actinomyces
- S – Streptococcus pneumoniae
- T – Treponema
5.4 Enrichment Media
Enrichment media are designed to favor the growth of specific microorganisms that may be present in small numbers within a mixed population. They are particularly useful for isolating pathogens from specimens with abundant normal flora.
Key Characteristics:
- Typically liquid media (broths)
- Contain nutrients or growth factors that promote growth of target organisms
- May contain inhibitory agents that suppress competing flora
- Used as a preliminary step before plating on selective or differential media
Common Examples:
- Selenite F Broth – For enrichment of Salmonella species
- Alkaline Peptone Water – For Vibrio species
- GN (Gram-Negative) Broth – For enteric pathogens
- Tetrathionate Broth – For Salmonella species
Note: It’s important to distinguish between “enriched” media (which contain additional nutrients for fastidious organisms) and “enrichment” media (which are designed to increase the relative numbers of specific organisms in a mixed sample).
5.5 Selective Media
Selective media contain specific inhibitory substances that suppress the growth of certain microorganisms while allowing others to grow. They are valuable for isolating specific pathogens from mixed specimens.
Key Characteristics:
- Contain selective agents (antibiotics, dyes, salts, etc.)
- Inhibit growth of unwanted microorganisms
- Allow growth of target organisms that are resistant to the selective agents
- Particularly useful for specimens from non-sterile sites
Common Examples:
| Selective Medium | Selective Agent | Target Organisms | Inhibited Organisms |
|---|---|---|---|
| MacConkey Agar | Bile salts, crystal violet | Gram-negative enteric bacteria | Gram-positive bacteria |
| Mannitol Salt Agar | 7.5% NaCl | Staphylococci | Most other bacteria |
| Thayer-Martin Agar | Antibiotics (vancomycin, colistin, nystatin) | Neisseria gonorrhoeae, N. meningitidis | Normal flora |
| TCBS Agar | Bile salts, citrate, thiosulfate | Vibrio species | Most enteric bacteria |
5.6 Differential Media
Differential media contain indicators that allow visual distinction between different types of microorganisms based on their metabolic characteristics. They help in preliminary identification of organisms based on colony appearance.
Key Characteristics:
- Contain indicators (e.g., pH indicators, precipitating agents)
- Allow visual differentiation of organisms based on biochemical reactions
- May use color changes, gas production, or precipitate formation
- Often used for preliminary identification
Common Examples:
| Differential Medium | Differential Characteristic | Visual Differentiation |
|---|---|---|
| Blood Agar | Hemolysis patterns | Alpha (green), Beta (clear), Gamma (no hemolysis) |
| MacConkey Agar | Lactose fermentation | Pink/red (lactose fermenters) vs. colorless (non-fermenters) |
| EMB Agar | Lactose/sucrose fermentation | Metallic sheen (E. coli) vs. pink/colorless (others) |
| TSI (Triple Sugar Iron) Agar | Fermentation of glucose, lactose, sucrose; H₂S production | Yellow/yellow, red/yellow, black precipitate |
| Mannitol Salt Agar | Mannitol fermentation | Yellow (S. aureus) vs. red/pink (S. epidermidis) |
Mnemonic: “DISC”
For remembering the characteristics of differential media:
- Detectable reactions (visible changes)
- Indicator compounds present
- Specific biochemical properties tested
- Color changes reveal metabolic activities
Note: Many media are both selective and differential. For example, MacConkey agar is selective for Gram-negative bacteria and differential for lactose fermentation.
Nursing Implementation
Understanding the types of culture media helps nurses in:
- Anticipating which specimens require special collection or transport media
- Interpreting preliminary laboratory findings based on media reactions
- Recognizing when certain pathogens might require specific media (e.g., Neisseria gonorrhoeae requires Thayer-Martin)
- Understanding the rationale behind specific specimen collection timing and techniques
6. Pure Culture Techniques
Pure culture techniques are methods used to isolate individual bacterial species from mixed populations. Obtaining pure cultures is essential for proper identification, susceptibility testing, and studying the characteristics of microorganisms.
6.1 Tube Dilution Method
The tube dilution method involves creating a series of dilutions of a bacterial suspension to reduce the number of organisms to a level where individual colonies can be isolated.
Procedure:
Applications:
- Quantitative analysis of bacterial populations
- Determining viable cell counts (CFU/ml)
- Preliminary step for pour plate and spread plate methods
6.2 Pour Plate Method
The pour plate method involves mixing a diluted bacterial suspension with molten agar and allowing it to solidify, resulting in colonies distributed throughout the medium as well as on the surface.
Procedure:
Advantages and Limitations:
| Advantages | Limitations |
|---|---|
|
|
6.3 Spread Plate Method
The spread plate method involves spreading a small volume of diluted bacterial suspension over the surface of a pre-poured agar plate, resulting in colonies that develop only on the surface.
Procedure:
Advantages and Limitations:
| Advantages | Limitations |
|---|---|
|
|
6.4 Streak Plate Method
The streak plate method is the most commonly used technique for isolating pure cultures. It involves progressively diluting bacteria over the surface of an agar plate by streaking with an inoculating loop.
Procedure:
Common Streak Plate Patterns:
- Four-quadrant streak – Divides plate into four sections with progressive dilution
- T-streak – Creates a T-pattern on the plate
- Continuous streak – Zigzag pattern across entire plate
Pro Tip: In clinical laboratories, the four-quadrant streak method is most commonly used as it provides a semi-quantitative assessment of bacterial growth (from 1+ to 4+) in addition to isolation of pure cultures.
Advantages:
- Simple technique requiring minimal equipment
- Highly effective for isolating pure cultures
- Allows observation of colony morphology
- No heat exposure to organisms
- Standard method in clinical laboratories
Mnemonic: “PSST”
For remembering pure culture techniques in order of increasing selectivity:
- Pour plate (least selective)
- Spread plate (moderately selective)
- Streak plate (highly selective)
- Tube dilution (used before plating)
Nursing Implementation
Knowledge of pure culture techniques helps nurses in:
- Understanding the importance of proper specimen collection to facilitate isolation of pathogens
- Recognizing why repeated cultures may be necessary when initial results show mixed growth
- Appreciating the time required for laboratory identification processes
- Explaining to patients why definitive results are not immediately available
- Understanding preliminary reports that describe colony types prior to final identification
7. Anaerobic Cultivation of Bacteria
Anaerobic bacteria are organisms that grow in the absence of oxygen. Some are obligate anaerobes (cannot tolerate oxygen at all), while others are facultative (can grow with or without oxygen). Cultivating anaerobes requires special techniques to create and maintain oxygen-free environments.
7.1 Methods for Creating Anaerobic Conditions
Common Techniques:
| Method | Description | Applications |
|---|---|---|
| Anaerobic Jars | Sealed containers with gas-generating sachets (e.g., GasPak) that consume O₂ and produce CO₂ | Small-scale laboratory use, routine clinical specimens |
| Anaerobic Chambers (Glove Boxes) | Sealed workstations with controlled atmosphere (N₂, CO₂, H₂) and airlocks | Research laboratories, reference centers, high-volume clinical work |
| Anaerobic Culture Systems | Commercial systems (e.g., PRAS, Anoxomat) with pre-reduced media and automated atmosphere generation | Clinical microbiology laboratories |
| Deep Agar Methods | Inoculation deep within agar tubes where oxygen diffusion is limited | Teaching laboratories, certain biochemical tests |
| Candle Jar | Sealed container where a burning candle consumes O₂ (creates microaerophilic conditions) | Cultivation of capnophilic organisms (e.g., Neisseria) |
Note: Anaerobic indicators (e.g., resazurin, methylene blue) are often used to confirm that anaerobic conditions have been achieved. These compounds change color when exposed to oxygen.
7.2 Specialized Anaerobic Media
Anaerobic media often contain reducing agents to remove trace oxygen and maintain a low oxidation-reduction potential favorable for anaerobic growth.
Key Components of Anaerobic Media:
- Reducing agents – Thioglycollate, cysteine, ascorbic acid
- Redox indicators – Resazurin (pink when oxidized, colorless when reduced)
- Oxygen scavengers – To remove trace oxygen
- Hemin and Vitamin K – Growth factors for many anaerobes
Common Anaerobic Media:
| Media | Composition | Purpose |
|---|---|---|
| Thioglycollate Broth | Casein, yeast extract, glucose, sodium thioglycollate, resazurin | General purpose medium for anaerobes; the reducing agent creates anaerobic conditions at the bottom of the tube |
| Brucella Blood Agar | Peptones, dextrose, yeast extract, sheep blood, vitamin K, hemin | Isolation and cultivation of fastidious anaerobes |
| CDC Anaerobe Blood Agar | Complex base with vitamin K, hemin, L-cystine, sheep blood | Isolation of obligate anaerobes from clinical specimens |
| Bacteroides Bile Esculin (BBE) Agar | Bile, esculin, gentamicin | Selective for Bacteroides fragilis group |
| PRAS (Pre-Reduced Anaerobically Sterilized) Media | Various formulations prepared, sterilized, and stored under anaerobic conditions | Cultivation of extremely oxygen-sensitive anaerobes |
Pre-Reduced Media Preparation:
Mnemonic: “ANAEROBES”
Key anaerobic bacteria of clinical importance:
- Actinomyces
- Non-sporing anaerobes (Prevotella, Porphyromonas)
- Anaerococcus
- Eubacterium
- Ruminococcus
- Obligate anaerobes (Bacteroides, Fusobacterium)
- Bacteroides fragilis group
- Epsilons (Clostridium)
- Spore-forming anaerobes (Clostridium tetani, C. perfringens)
Specimen Collection for Anaerobic Culture:
Proper collection of specimens for anaerobic culture is critical as exposure to oxygen can kill sensitive anaerobes.
- Use anaerobic transport media or systems
- Avoid contamination with normal flora
- Obtain adequate sample volume
- Transport specimens promptly to the laboratory
- Avoid needle aspiration if possible (use anaerobic collection systems)
Nursing Implementation
Nurses play a crucial role in the proper collection of specimens for anaerobic culture:
- Ensure proper specimen collection techniques to minimize oxygen exposure
- Use appropriate anaerobic transport systems
- Transport specimens promptly to the laboratory
- Document relevant clinical information (antibiotic therapy, site of collection, suspected diagnosis)
- Recognize clinical presentations suggestive of anaerobic infections (foul odor, gas production, tissue necrosis)
- Understand that anaerobic cultures require longer incubation periods (results may take 48-72 hours or longer)
Clinical Scenarios Requiring Anaerobic Culture:
- Deep wound infections
- Abscesses (brain, liver, lung, etc.)
- Aspiration pneumonia
- Peritonitis
- Pelvic inflammatory disease
- Necrotizing fasciitis
- Gangrene
8. Nursing Implications in Clinical Microbiology
Nurses play a critical role in the microbiological diagnosis process. Understanding culture media and techniques helps nurses improve specimen quality, interpret results, and provide better patient care.
Key Nursing Responsibilities:
- Specimen Collection – Using proper technique to obtain high-quality specimens
- Specimen Handling – Ensuring proper transport and storage conditions
- Documentation – Recording relevant clinical information (antibiotic use, suspected diagnosis)
- Result Interpretation – Understanding culture reports and their implications for patient care
- Infection Control – Implementing appropriate precautions based on microbiological findings
- Patient Education – Explaining procedures and the importance of appropriate specimen collection
Clinical Applications of Microbiological Knowledge:
| Scenario | Nursing Actions | Microbiology Considerations |
|---|---|---|
| Suspected UTI | Clean-catch midstream urine collection, prompt transport to lab | CLED or MacConkey agar; quantitative culture to distinguish contamination from infection |
| Wound infection | Proper cleaning before collection, deep tissue sampling when possible | Both aerobic and anaerobic cultures; blood agar and selective media |
| Suspected sepsis | Collecting multiple blood culture sets, proper skin antisepsis | Blood culture broths with resins or activated charcoal to neutralize antibiotics |
| Respiratory infection | Collecting sputum (not saliva), instructing on deep cough technique | Selective media for respiratory pathogens; Gram stain to assess specimen quality |
| Suspected TB | Collection of early morning sputum, airborne precautions | Specialized media (Lowenstein-Jensen); acid-fast staining |
Important: Always collect specimens for culture before initiating antimicrobial therapy whenever possible. If the patient is already on antibiotics, this should be clearly documented on the laboratory requisition.
9. Quality Control in Media Preparation
Quality control procedures ensure that culture media perform consistently and reliably. Both commercially prepared and laboratory-prepared media must undergo quality checks before use.
Quality Control Parameters:
- Physical characteristics – Appearance, consistency, color, hemolysis
- Chemical characteristics – pH, moisture content, sterility
- Biological characteristics – Growth support, selectivity, biochemical reactions
Quality Control Testing Procedures:
- Sterility Check – Incubation of uninoculated media to ensure absence of contamination
- Growth Support – Inoculation with standard organisms to verify growth promotion
- Selectivity Testing – Verifying that selective media inhibit appropriate organisms
- Biochemical Reaction Testing – Confirming that differential media produce expected reactions
- pH Verification – Ensuring proper pH after sterilization
The Clinical and Laboratory Standards Institute (CLSI) provides guidelines for quality control in microbiology laboratories, including recommendations for testing culture media.
10. Conclusion
Understanding culture media preparation and bacterial cultivation techniques is fundamental for nursing students engaged in clinical microbiology. This knowledge supports better specimen collection, improved communication with laboratory professionals, and enhanced patient care through accurate interpretation of microbiological findings.
Key concepts to remember include:
- The basic principles of media preparation and sterilization
- The differences between solid and liquid media and their applications
- The various types of media (semi-synthetic, synthetic, enriched, enrichment, selective, differential) and their purposes
- Pure culture techniques and their appropriate applications
- Special considerations for anaerobic cultivation
- The critical role of nurses in the specimen collection process
By applying this knowledge in clinical practice, nurses can contribute significantly to the accurate and timely diagnosis of infectious diseases, leading to more effective treatment and better patient outcomes.