Care of Equipment: Decontamination and Disposal
Complete Guide for Syringes, Needles, and Infusion Sets
Table of Contents
Introduction to Equipment Care
Why This Matters
Proper decontamination and disposal of medical equipment is the cornerstone of infection prevention in healthcare settings. Every year, healthcare-associated infections affect millions of patients worldwide, with improper equipment handling being a significant contributing factor.
Patient Safety Impact
Reduces risk of healthcare-associated infections by 85% when protocols are followed correctly
Healthcare Worker Protection
Prevents needlestick injuries and bloodborne pathogen exposure for medical staff
In the modern healthcare environment, the proper care of medical equipment through effective decontamination processes has become more critical than ever. This comprehensive guide will equip nursing students and professionals with the essential knowledge and skills needed to handle syringes, needles, and infusion sets safely and effectively.
The decontamination process involves multiple steps that must be executed with precision and attention to detail. Understanding these procedures not only ensures patient safety but also protects healthcare workers from potential exposure to infectious agents.
Proper Decontamination Process Overview
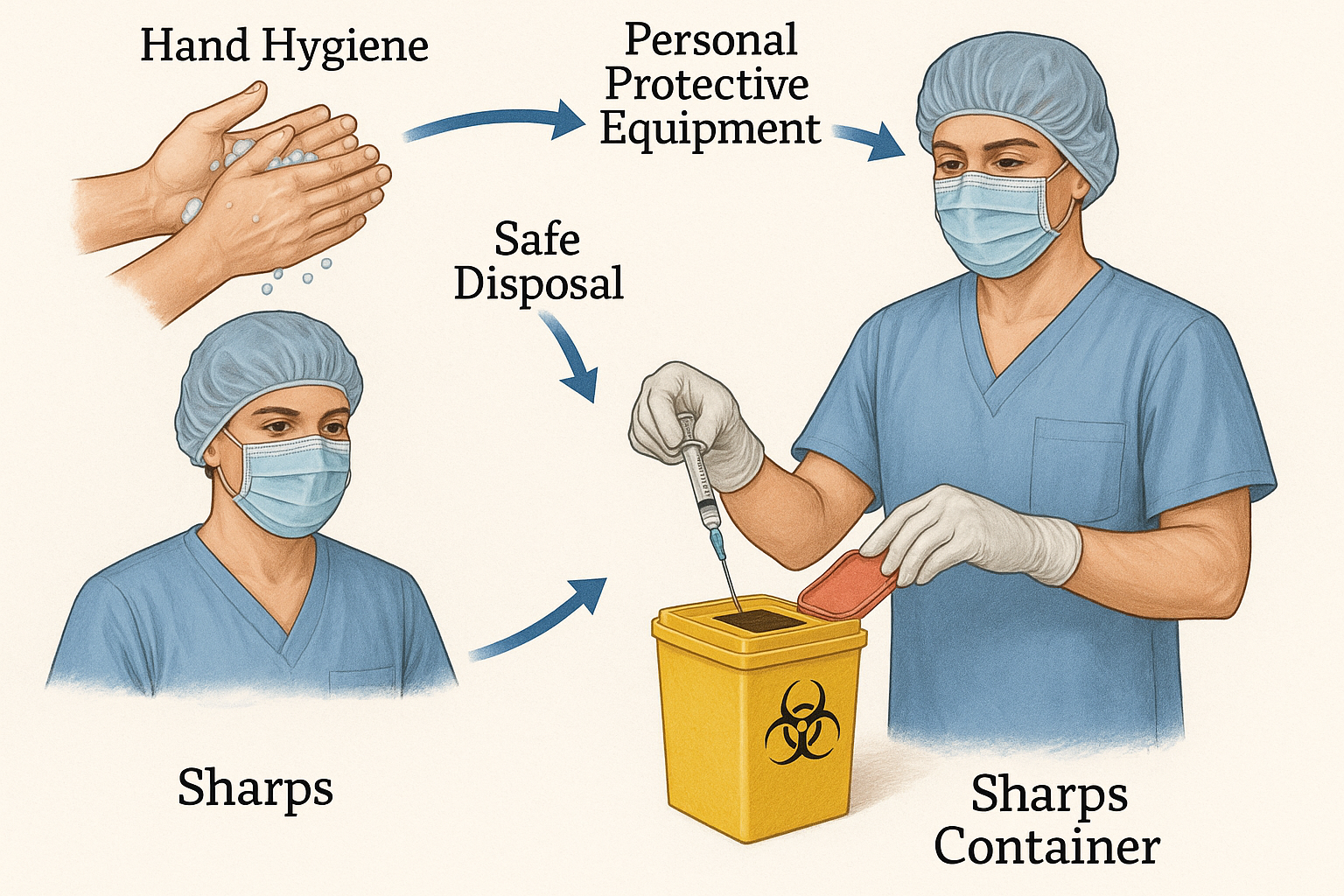
Visual guide showing the complete decontamination and disposal process for medical equipment
Fundamentals of Decontamination
Sterilization
Complete elimination of all microorganisms including spores
Disinfection
Reduction of pathogenic microorganisms to safe levels
Cleaning
Physical removal of visible soil and debris
The Decontamination Hierarchy
| Level | Process | Equipment Type | Method |
|---|---|---|---|
| Critical | Sterilization | Syringes, Needles | Single-use disposal |
| Semi-critical | High-level disinfection | Infusion pump surfaces | Chemical disinfection |
| Non-critical | Low-level disinfection | External equipment surfaces | Routine cleaning |
Memory Aid: CLEAN Method
- C – Collect contaminated equipment safely
- L – Label and separate by contamination level
- E – Execute proper decontamination procedure
- A – Apply appropriate disposal method
- N – Note and document the process
The decontamination process begins the moment equipment becomes contaminated with patient materials. Understanding the different levels of decontamination ensures that each piece of equipment receives appropriate treatment based on its risk category and intended use.
Syringe Care and Disposal
Critical Safety Note
All syringes are considered single-use items and must NEVER be reused, regardless of cleaning or decontamination attempts.
Types of Syringes and Their Classifications
Standard Syringes
1-60mL capacity, Luer-lock or slip-tip
Risk: High contamination potential
Safety Syringes
Built-in needle protection mechanism
Risk: Reduced needlestick injury
Pre-filled Syringes
Manufacturer-filled with medication
Risk: Medication contamination
Proper Syringe Disposal Protocol
-
1
Immediate Post-Use Assessment
Do not recap needles. Keep syringe and needle assembly intact for disposal.
-
2
Sharps Container Placement
Place entire syringe-needle unit directly into approved sharps container immediately after use.
-
3
Hand Hygiene
Perform immediate hand hygiene with alcohol-based hand rub or soap and water.
Time-Critical Actions
Syringe disposal must occur within 60 seconds of use to minimize contamination risk. Any delay increases the potential for accidental needlestick injuries and cross-contamination.
The decontamination approach for syringes differs significantly from reusable medical equipment. Since syringes are single-use items, the focus shifts from cleaning and sterilization to immediate, safe disposal following established protocols.
Needle Safety Management
Never Do These Actions
- Recap used needles
- Bend or break needles
- Remove needles from syringes
- Place needles in regular trash
Always Do These Actions
- Dispose immediately after use
- Use sharps containers only
- Activate safety features
- Report exposure incidents
Needle Classification System
| Needle Type | Gauge Range | Primary Use | Disposal Priority |
|---|---|---|---|
| Hypodermic | 16G – 30G | Injections, blood draws | Immediate |
| IV Catheter | 14G – 24G | Intravenous access | Immediate |
| Butterfly | 21G – 25G | Pediatric, difficult access | Immediate |
| Spinal | 18G – 27G | Lumbar puncture | Immediate |
Memory Aid: SHARP Protocol
- S – See the sharps container before use
- H – Handle with extreme caution
- A – Activate safety mechanisms immediately
- R – Remove from patient safely
- P – Place directly in sharps container
Needlestick Injury Prevention
Statistical Reality: Healthcare workers experience approximately 385,000 needlestick injuries annually in the United States alone. Proper decontamination protocols and immediate disposal are critical prevention measures.
Effective needle safety management represents a cornerstone of healthcare worker protection and patient safety. The decontamination process for needles focuses primarily on immediate, safe disposal rather than cleaning or sterilization, as needles are single-use medical devices designed for one-time application.
Infusion Set Protocols
Primary Sets
72-96 hour change
Secondary Sets
24-hour change
Blood Products
4-hour maximum
Lipid Emulsions
12-24 hour change
Infusion Set Component Analysis
Spike/Connector
First point of contamination risk
Tubing
Primary fluid pathway
Ports/Injection Sites
Multiple access points
Decontamination Schedule Matrix
| Infusion Type | Change Frequency | Decontamination Method | Disposal Classification |
|---|---|---|---|
| Continuous IV Fluids | Every 72-96 hours | Complete set replacement | Regular medical waste |
| Intermittent Medications | Every 24 hours | Port disinfection + set change | Regular medical waste |
| Blood/Blood Products | After each unit (max 4h) | Immediate disposal | Biohazard waste |
| Chemotherapy | Immediately after use | Hazmat protocols | Hazardous waste |
Best Practice Reminder
Infusion set decontamination involves both time-based and event-based protocols. The key is maintaining sterility throughout the infusion process while ensuring timely replacement to prevent biofilm formation and bacterial growth.
Unlike syringes and needles, infusion sets require ongoing monitoring and scheduled decontamination procedures. The complexity of infusion set management stems from their extended use periods and multiple access points, making proper decontamination protocols essential for preventing catheter-related bloodstream infections.
Step-by-Step Decontamination Procedures
Procedure 1: Single-Use Item Disposal
Pre-Disposal Assessment (30 seconds)
- 1 Identify item contamination level
- 2 Locate appropriate disposal container
- 3 Verify PPE integrity and placement
Safe Disposal Execution (60 seconds)
- 4 Activate safety mechanisms if present
- 5 Place item directly into container
- 6 Perform immediate hand hygiene
Procedure 2: Infusion Set Decontamination
Phase 1: Preparation
- • Gather replacement equipment
- • Apply appropriate PPE
- • Stop infusion temporarily
- • Clamp all access points
Phase 2: Disconnection
- • Disinfect connection points
- • Remove old infusion set
- • Cap exposed ports immediately
- • Handle contaminated items minimally
Phase 3: Installation
- • Prime new tubing system
- • Connect using sterile technique
- • Resume infusion as prescribed
- • Document replacement time
Procedure 3: Contaminated Equipment Management
High-Risk Scenario Protocol
For equipment with visible blood contamination or suspected infectious material exposure
Immediate Actions
Decontamination Sequence
Universal Procedure Memory Aid: DISPOSE
- D – Determine contamination level
- I – Isolate contaminated equipment
- S – Select appropriate disposal method
- P – Protect yourself with PPE
- O – Operate according to protocol
- S – Sanitize hands immediately
- E – Execute documentation requirements
Safety Protocols and Personal Protective Equipment
Standard Precautions
- • Hand hygiene before/after contact
- • Gloves for potential exposure
- • Eye protection if splash risk
- • Appropriate waste segregation
Contact Precautions
- • Enhanced glove usage
- • Dedicated equipment when possible
- • Extended decontamination time
- • Additional surface disinfection
Bloodborne Pathogen
- • Double gloving recommended
- • Face shield or goggles
- • Fluid-resistant gown
- • Immediate exposure reporting
PPE Selection Matrix for Decontamination Tasks
| Equipment Type | Minimum PPE | Enhanced PPE | Special Considerations |
|---|---|---|---|
| Clean Syringes | Gloves | Gloves + Eye Protection | Consider medication residue |
| Used Needles | Gloves + Eye Protection | Double Gloves + Face Shield | Never recap or manipulate |
| Blood-contaminated Items | Full PPE Suite | Enhanced + Shoe Covers | Biohazard protocols apply |
| Infusion Sets | Gloves + Gown | Standard + Eye Protection | Medication-specific requirements |
Hand Hygiene Protocol Enhancement
Alcohol-Based Hand Rub
When to Use: Hands not visibly soiled
Duration: 20-30 seconds until dry
Volume: 3-5mL (palm-full)
Effectiveness: 99.9% pathogen reduction
Soap and Water
When to Use: Visible soiling or contamination
Duration: Minimum 40-60 seconds
Temperature: Warm water preferred
Technique: Mechanical action essential
Critical Safety Insight
The decontamination process effectiveness is directly proportional to the quality of PPE usage and adherence to safety protocols. Studies show that proper PPE use reduces healthcare worker infection risk by over 90% during equipment decontamination procedures.
PPE Memory Aid: PROTECT
- P – Plan PPE selection before starting
- R – Remove contaminated items safely
- O – Organize work area for efficiency
- T – Time procedures to minimize exposure
- E – Evaluate contamination level continuously
- C – Clean hands immediately after removal
- T – Track exposure incidents for reporting
Infection Control Measures
Primary Objectives of Infection Control
Protect Patients
Prevent healthcare-associated infections
Protect Healthcare Workers
Minimize occupational exposure risk
Protect Environment
Prevent environmental contamination
Chain of Infection Interruption
Pathogen
Bacteria, viruses, fungi
Reservoir
Equipment surfaces
Portal of Exit
Blood, body fluids
Transmission
Contact, airborne
Portal of Entry
Wounds, mucous membranes
Susceptible Host
Patients, staff
Decontamination Impact on Chain of Infection
Proper decontamination procedures break the chain of infection at multiple points: eliminating pathogens from equipment (reservoir), preventing transmission routes, and reducing environmental contamination.
Environmental Decontamination Zones
High-Risk Zone
- • Patient bed area (3-foot radius)
- • Equipment contact surfaces
- • Bedside tables and rails
- • IV poles and pumps
Moderate-Risk Zone
- • Room furniture and fixtures
- • Door handles and light switches
- • Computer keyboards and monitors
- • Storage areas
Low-Risk Zone
- • Hallways and corridors
- • Administrative areas
- • Staff break rooms
- • General storage
Decontamination Agent Selection
| Agent Type | Active Ingredient | Spectrum | Contact Time | Best Use |
|---|---|---|---|---|
| Alcohol-based | 70% Isopropanol | Broad spectrum | 30 seconds | Equipment surfaces |
| Chlorine-based | Sodium hypochlorite | Broad + sporicidal | 1-10 minutes | Blood spills |
| Quaternary ammonium | Benzalkonium chloride | Moderate spectrum | 2-5 minutes | Environmental surfaces |
| Hydrogen peroxide | H2O2 accelerated | Broad + sporicidal | 0.5-3 minutes | Critical equipment |
Infection Control Memory Aid: CONTROL
- C – Classify contamination risk level
- O – Organize decontamination workflow
- N – Neutralize pathogens effectively
- T – Time contact periods appropriately
- R – Remove contaminated materials safely
- O – Observe standard precautions
- L – Log decontamination activities
Documentation and Compliance
Legal and Regulatory Framework
Proper documentation of decontamination procedures is not just best practice—it’s a legal requirement under multiple regulatory bodies including OSHA, CDC, and Joint Commission standards.
Legal Protection
Accreditation
Quality Improvement
Essential Documentation Elements
Required Documentation Fields
- • Date and time of decontamination
- • Equipment type and identification
- • Decontamination method used
- • Staff member responsible
- • Patient/case reference number
- • Disposal method and location
- • Any incidents or deviations
- • Quality assurance verification
Critical Incident Documentation
- • Needlestick or sharps injuries
- • Equipment malfunction during use
- • Contamination exposure events
- • Protocol deviations or violations
- • Equipment damage or loss
- • Adverse patient reactions
- • Environmental contamination
- • Staff training deficiencies
Documentation Workflow
Pre-Procedure
Equipment assessment and preparation documentation
During Procedure
Real-time monitoring and deviation recording
Post-Procedure
Completion verification and outcome documentation
Follow-up
Quality assurance review and corrective actions
Compliance Monitoring Metrics
| Metric Category | Measurement | Target Standard | Monitoring Frequency |
|---|---|---|---|
| Protocol Adherence | % of procedures following standard | ≥95% | Monthly audit |
| Documentation Completeness | % of required fields completed | 100% | Weekly review |
| Incident Reporting | Time to report (hours) | ≤24 hours | Per incident |
| Staff Competency | Training completion rate | 100% | Annual assessment |
Documentation Red Flags
Common Documentation Failures:
- • Missing timestamps or dates
- • Incomplete staff signatures
- • Vague procedure descriptions
- • Delayed incident reporting
Compliance Consequences:
- • Regulatory penalties and fines
- • Accreditation jeopardy status
- • Increased liability exposure
- • Quality rating impacts
Documentation Memory Aid: RECORD
- R – Record immediately after completion
- E – Ensure all required fields are complete
- C – Check accuracy and legibility
- O – Obtain appropriate signatures
- R – Report incidents within timeframes
- D – Date and timestamp all entries
Emergency Decontamination Procedures
Time-Critical Response Framework
Emergency decontamination procedures must be executed within specific timeframes to minimize harm. The “Golden Hour” principle applies to exposure incidents, where immediate action significantly improves outcomes.
Immediate Response
Secure area, assess exposure
Emergency Decontamination
Execute decontamination protocol
Medical Evaluation
Medical assessment, treatment
Needlestick Injury Emergency Protocol
Immediate Actions (0-2 minutes)
- 1 Remove gloves carefully to avoid further contamination
- 2 Allow wound to bleed freely for 10-15 seconds
- 3 Wash with soap and warm water for 2 minutes minimum
- 4 Apply antiseptic (70% alcohol or iodine solution)
Reporting Actions (2-10 minutes)
- 1 Notify immediate supervisor or charge nurse
- 2 Contact occupational health or emergency department
- 3 Preserve source equipment for testing if possible
- 4 Begin incident documentation immediately
Chemical/Biological Spill Emergency
Spill Response Hierarchy
Minor Spill
{‘<'}10mL, no aerosol generation
Major Spill
10-100mL, potential aerosol
Massive Spill
{‘>’}100mL, widespread contamination
Equipment Malfunction During Decontamination
| Malfunction Type | Immediate Response | Safety Priority | Next Steps |
|---|---|---|---|
| Sharps container overflow | Stop all sharp disposals immediately | Prevent needlestick injuries | Replace container, secure overfilled unit |
| Autoclave failure | Quarantine unprocessed items | Prevent use of non-sterile equipment | Alternative sterilization method |
| Disinfectant shortage | Implement alternative protocol | Maintain decontamination standards | Emergency supply procurement |
| PPE supply depletion | Restrict non-essential procedures | Protect healthcare workers | Emergency supply chain activation |
Emergency Response Memory Aid: EMERGENCY
- E – Evaluate situation immediately
- M – Minimize further exposure risk
- E – Execute appropriate protocol
- R – Report to supervisory staff
- G – Get medical evaluation if needed
- E – Ensure proper documentation
- N – Notify all relevant parties
- C – Clean and decontaminate thoroughly
- Y – Yield to expert guidance when needed
Global Best Practices in Equipment Decontamination
International Excellence in Decontamination Standards
Healthcare systems worldwide have developed innovative approaches to equipment decontamination, incorporating advanced technology, rigorous protocols, and continuous quality improvement. These practices demonstrate the global commitment to patient safety and healthcare worker protection.
Canada: Comprehensive Sharps Safety Program
Key Innovations:
- • Mandatory safety-engineered sharps devices in all healthcare settings
- • Real-time electronic tracking of sharps disposal
- • Integrated injury surveillance and prevention programs
- • Standardized provincial protocols across all facilities
Measurable Outcomes:
- • 85% reduction in needlestick injuries since implementation
- • 99.7% compliance rate with disposal protocols
- • Significant cost savings in post-exposure management
- • Enhanced staff satisfaction and safety perception
United Kingdom: NHS Digital Decontamination Initiative
Digital Integration:
- • RFID-enabled equipment tracking throughout decontamination cycle
- • Automated documentation and compliance monitoring
- • Machine learning algorithms for predictive maintenance
- • Mobile apps for real-time procedure guidance
Quality Assurance:
- • Continuous environmental monitoring systems
- • Blockchain-secured audit trails for accountability
- • AI-powered anomaly detection in processes
- • Patient outcome correlation analysis
Australia: Zero Waste Healthcare Initiative
Sustainability Focus:
- • Advanced autoclave systems with energy recovery
- • Biodegradable packaging for single-use items
- • Regional centralized decontamination facilities
- • Comprehensive recycling programs for equipment components
Environmental Impact:
- • 40% reduction in medical waste generation
- • Carbon footprint reduction of 25% per facility
- • Water conservation through closed-loop systems
- • Community engagement in environmental health
Switzerland: Precision Decontamination Standards
Technical Excellence:
- • Pharmaceutical-grade air filtration in decontamination areas
- • Multi-parameter validation for each decontamination cycle
- • Precision temperature and humidity control systems
- • Advanced chemical indicators for process verification
Staff Excellence:
- • Certified decontamination technician programs
- • Continuous competency assessment protocols
- • International collaboration and knowledge exchange
- • Research partnerships with academic institutions
Japan: Robotics-Enhanced Decontamination
Technological Innovation:
- • Robotic sorting and handling of contaminated equipment
- • UV-C light disinfection robots for environmental surfaces
- • Automated inventory management for decontamination supplies
- • Artificial intelligence-guided protocol optimization
Safety Enhancement:
- • Minimized human contact with contaminated materials
- • 24/7 continuous decontamination capability
- • Reduced physical strain on healthcare workers
- • Enhanced precision in hazardous material handling
Netherlands: Integrated Healthcare Sustainability
Circular Economy Principles:
- • Reusable equipment design partnerships with manufacturers
- • Advanced material recovery from medical waste streams
- • Life cycle assessment for all decontamination processes
- • Regional cooperation for resource optimization
Innovation Outcomes:
- • 60% reduction in single-use equipment consumption
- • Development of next-generation reusable devices
- • Cost savings reinvested in patient care improvements
- • International model for sustainable healthcare
Key Learning Points from Global Excellence
Technology Integration:
Advanced technology enhances safety, efficiency, and compliance while reducing human error in decontamination processes.
Sustainability Focus:
Environmental responsibility and economic efficiency can be achieved simultaneously through innovative decontamination approaches.
Staff Development:
Continuous education and competency development are essential for maintaining high decontamination standards.
Quality Assurance:
Comprehensive monitoring and evaluation systems ensure consistent adherence to best practices and continuous improvement.
Conclusion and Key Takeaways
Mastery of Decontamination Excellence
The proper care of medical equipment through effective decontamination and disposal represents one of the most critical competencies in modern nursing practice. This comprehensive guide has provided you with the essential knowledge, skills, and understanding necessary to excel in this vital area of patient care.
Essential Competencies Achieved
Technical Proficiency
Understanding of decontamination principles, equipment classification, and proper disposal methods for all medical devices.
Safety Expertise
Comprehensive knowledge of PPE requirements, exposure prevention, and emergency response protocols.
Compliance Mastery
Thorough understanding of documentation requirements, regulatory standards, and quality assurance measures.
Infection Control
Advanced knowledge of infection prevention strategies and environmental decontamination protocols.
Emergency Response
Competency in handling emergency decontamination situations and exposure incident management.
Best Practice Integration
Awareness of global standards and innovative approaches to equipment decontamination.
Critical Success Factors
Vigilant Observation
Continuous monitoring of equipment condition and contamination status
Timely Execution
Adherence to prescribed timeframes for all decontamination procedures
Precision Technique
Meticulous attention to detail in every step of the process
Continuous Improvement
Ongoing learning and adaptation to new standards and technologies
Future Directions in Equipment Decontamination
Emerging Trends and Technologies
The field of medical equipment decontamination continues to evolve with advances in nanotechnology, artificial intelligence, and sustainable healthcare practices. Future nursing professionals must remain adaptable and committed to lifelong learning.
Final Memory Aid: EXCELLENCE
- E – Execute protocols with precision
- X – X-ray vision for detail attention
- C – Continuous learning and improvement
- E – Excellence in every procedure
- L – Leadership in safety practices
- L – Lifelong commitment to standards
- E – Efficiency without compromise
- N – Never accept substandard practices
- C – Compassionate patient care focus
- E – Exemplary professional behavior
Your Journey Forward
As you progress in your nursing career, remember that mastery of decontamination procedures is not just about following protocols—it’s about protecting lives, preventing infections, and contributing to the highest standards of healthcare delivery.
Every piece of equipment you handle safely, every protocol you follow precisely, and every colleague you educate contributes to a safer healthcare environment for all. Your commitment to excellence in decontamination practices makes you an invaluable member of the healthcare team.
