Neonatal Infections
Comprehensive Nursing Notes
Table of Contents
1. Introduction
Neonatal infections remain a significant cause of morbidity and mortality in newborns worldwide. These infections can be acquired at different times: in utero (congenital), during delivery (intrapartum), or after birth (postnatal). Understanding the pathophysiology, clinical presentation, diagnosis, and management of neonatal infections is crucial for providing optimal care to these vulnerable patients.
Epidemiology:
- Neonatal infections affect approximately 1-10 per 1000 live births in developed countries
- This rate is significantly higher in developing countries (10-50 per 1000 live births)
- They account for approximately 15-30% of neonatal deaths worldwide
- Early-onset sepsis (EOS) has a mortality rate of 3-40%, while late-onset sepsis (LOS) has a mortality rate of 2-20%
As a nursing professional, your role in the identification, prevention, and management of neonatal infections is critical. This comprehensive guide will equip you with the knowledge and tools necessary to provide evidence-based care to newborns at risk for or experiencing infections.
2. Classification of Neonatal Infections
Timing-Based Classification
(0-72 hours)
(4-28 days)
(>28 days)
| Classification | Timing | Common Pathogens | Mode of Transmission |
|---|---|---|---|
| Early-Onset Sepsis (EOS) | 0-72 hours after birth | Group B Streptococcus (GBS), Escherichia coli, Listeria monocytogenes | Vertical transmission from mother (transplacental, ascending, or during delivery) |
| Late-Onset Sepsis (LOS) | 4-28 days after birth | Coagulase-negative Staphylococci, Staphylococcus aureus, Gram-negative bacilli, Candida species | Horizontal transmission from environment or healthcare providers |
| Very Late-Onset Sepsis | >28 days (relevant in NICU) | Similar to LOS, with higher fungal incidence | Horizontal transmission, often associated with prolonged hospitalization |
TORCH Infections

TORCH is an acronym that refers to a group of congenital infections that can occur when a pregnant woman contracts certain infections that can pass through the placenta to the fetus. If not properly managed, these infections can lead to severe birth defects or fetal/neonatal death.
TORCH Mnemonic
| TORCH Infection | Key Clinical Features | Diagnosis | Management |
|---|---|---|---|
| Toxoplasmosis | Hydrocephalus, intracranial calcifications, chorioretinitis, hepatosplenomegaly | Maternal & neonatal serology, PCR of amniotic fluid | Spiramycin (maternal); Pyrimethamine, sulfadiazine & leucovorin (infant) |
| Other: Syphilis | Rash, hepatosplenomegaly, jaundice, rhinitis, osteochondritis | VDRL, RPR, FTA-ABS | Penicillin G |
| Rubella | Cataracts, hearing loss, PDA, microcephaly, “blueberry muffin” rash | Rubella-specific IgM antibodies, viral culture | Supportive care (no specific antiviral therapy) |
| Cytomegalovirus | Microcephaly, periventricular calcifications, sensorineural hearing loss, chorioretinitis | PCR of urine, saliva, or CSF | Ganciclovir for symptomatic disease |
| Herpes Simplex | Vesicular skin lesions, keratoconjunctivitis, seizures, lethargy, DIC | PCR of lesions, CSF; viral culture | Acyclovir |
Pathogen-Based Classification
3. Pathophysiology
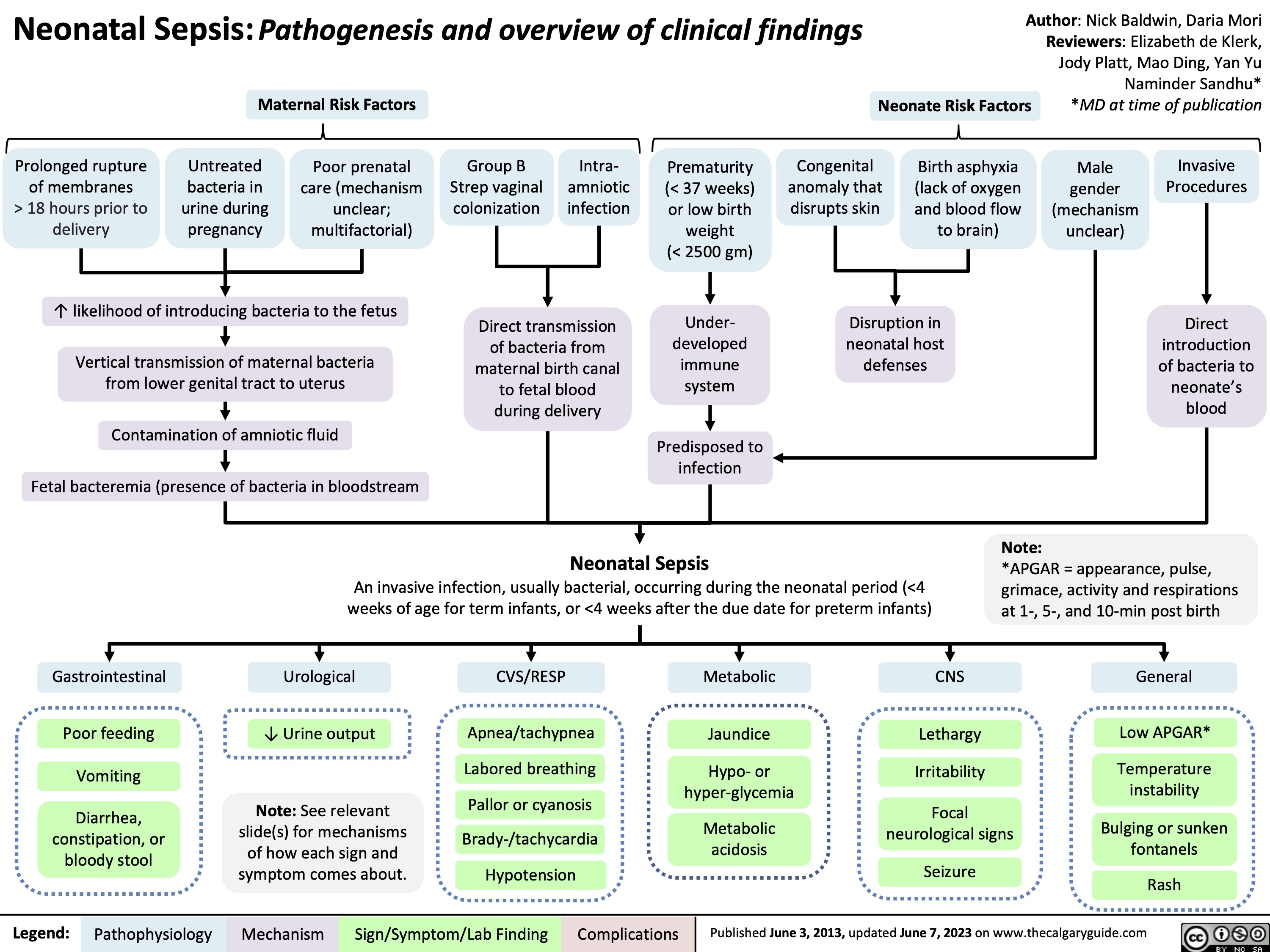
Neonatal Immune System Immaturity
Neonates are particularly susceptible to infections due to several factors related to their immature immune system:
| Immune Component | Deficiency in Neonates | Clinical Implication |
|---|---|---|
| Physical Barriers | Thin skin, immature mucosal barriers | Easier pathogen penetration |
| Complement System | Reduced complement levels (50-75% of adult levels) | Decreased opsonization and bacterial killing |
| Neutrophils | Reduced chemotaxis, phagocytosis, and oxidative burst | Compromised bacterial clearance |
| Immunoglobulins | Limited IgM, IgA production; IgG is maternal-derived | Reduced humoral immunity, especially against gram-negative bacteria |
| Cytokine Production | Imbalanced Th1/Th2 response, favoring Th2 | Decreased cell-mediated immunity |
Pathways of Infection Acquisition
Progression from Infection to Sepsis
The development of sepsis in neonates follows a cascade of events:
- Invasion: Pathogens breach the first-line defense barriers
- Recognition: Pathogen-associated molecular patterns (PAMPs) are recognized by pattern recognition receptors (PRRs)
- Inflammatory Response: Activation of pro-inflammatory cytokines (TNF-α, IL-1, IL-6, IL-8)
- Neutrophil Activation: Recruitment of neutrophils to infection site
- Endothelial Damage: Vascular permeability increases, leading to extravasation of fluid
- Coagulation Abnormalities: Activation of coagulation cascade, potentially leading to DIC
- Organ Dysfunction: Circulatory failure, tissue hypoxia, and multi-organ dysfunction
- Septic Shock: Cardiovascular compromise with inadequate tissue perfusion despite adequate fluid resuscitation
Important Risk Factors for Neonatal Infections:
- Maternal factors: Group B Streptococcal colonization, chorioamnionitis, PROM >18 hours, intrapartum fever
- Neonatal factors: Prematurity, low birth weight, immunodeficiency, congenital anomalies
- Procedural factors: Invasive procedures, central venous catheters, endotracheal intubation, prolonged hospitalization
4. Clinical Manifestations
Neonatal infections often present with nonspecific signs and symptoms, making early diagnosis challenging. The clinical manifestations can vary based on the timing of infection, causative organism, and severity of infection.
Mnemonic: “SEPTIC NEONATE”
System-Specific Manifestations
| System | Clinical Manifestations |
|---|---|
| Respiratory | Tachypnea, grunting, nasal flaring, retractions, apnea, cyanosis, increased oxygen requirement |
| Cardiovascular | Tachycardia, bradycardia, poor peripheral perfusion, hypotension, mottled skin, prolonged capillary refill |
| Gastrointestinal | Feeding intolerance, abdominal distention, vomiting, diarrhea, hepatomegaly, jaundice, necrotizing enterocolitis (NEC) |
| Neurological | Lethargy, irritability, poor tone, hyporeflexia, seizures, bulging fontanelle, high-pitched cry |
| Hematological | Pallor, petechiae, purpura, bleeding, hepatosplenomegaly, disseminated intravascular coagulation (DIC) |
| Metabolic | Temperature instability (fever or hypothermia), glucose instability, metabolic acidosis |
| Skin | Pustules, erythema, cellulitis, omphalitis (umbilical infection), “blueberry muffin” rash (in TORCH infections) |
Manifestations Specific to Causative Pathogens
Group B Streptococcus (GBS)
- Predominantly early-onset disease
- Respiratory distress within hours of birth
- Rapid progression to pneumonia and septic shock
- Can cause meningitis in late-onset disease
Escherichia coli
- Major cause of early and late-onset sepsis
- Predilection for meningitis, especially K1 strain
- Urinary tract infections in late-onset disease
- Higher mortality compared to GBS sepsis
Herpes Simplex Virus (HSV)
- Vesicular skin lesions (may be absent in 20%)
- Keratoconjunctivitis
- Encephalitis with seizures
- Disseminated disease with hepatitis and DIC
Cytomegalovirus (CMV)
- Microcephaly with periventricular calcifications
- Chorioretinitis
- Sensorineural hearing loss
- Hepatosplenomegaly with jaundice
- Petechiae (“blueberry muffin” rash)
Red Flags – Seek Immediate Medical Attention:
- Lethargy or poor responsiveness
- Refusal to feed or marked feeding intolerance
- Respiratory distress (severe retractions, grunting, apnea)
- Seizures
- Bulging fontanelle
- Temperature instability (>38°C or <36°C)
- Signs of shock (poor perfusion, hypotension)
- Purpuric rash
5. Diagnostic Approach
Early and accurate diagnosis of neonatal infections is crucial for timely intervention and improved outcomes. The diagnostic approach includes a combination of clinical assessment, laboratory investigations, and microbiological studies.
Clinical Assessment
A thorough clinical assessment should include:
- Complete maternal history (risk factors for infection)
- Perinatal history (mode of delivery, complications)
- Detailed physical examination
- Assessment of vital signs and general appearance
- System-specific examination for signs of infection
Laboratory Investigations
| Test | Findings in Infection | Limitations |
|---|---|---|
| Complete Blood Count (CBC) |
|
Low sensitivity and specificity; affected by gestational age and physiologic changes after birth |
| Blood Culture | Growth of pathogen (gold standard) | Takes time (24-72 hours); can be affected by maternal antibiotics; false negatives with small blood volumes |
| C-Reactive Protein (CRP) | >10 mg/L (serial measurements more useful than single value) | Rises 6-8 hours after infection; peaks at 24-48 hours; non-specific |
| Procalcitonin | Elevated (>2 ng/mL highly suggestive) | Physiologically elevated in first 48 hours of life; affected by stress and non-infectious conditions |
| Cerebrospinal Fluid (CSF) |
|
Traumatic tap can complicate interpretation; LP may be deferred in unstable neonates |
| Urine Culture | Growth of pathogen (>10,000 CFU/mL) | Mainly useful for late-onset sepsis; suprapubic aspiration or catheterization required |
Diagnostic Algorithm
Approach to Suspected Neonatal Sepsis
Clinical Signs Suggestive of Sepsis
Complete Septic Workup
CBC, Blood Culture, CRP
Consider: LP, Urine Culture, CXR
Low Suspicion
Normal CBC/CRP
Minimal clinical signs
Observe closely
Repeat labs in 6-12 hours
Consider stopping antibiotics at 36-48 hours if cultures negative
High Suspicion
Abnormal CBC/CRP
Significant clinical signs
Positive cultures
Treat for 7-14 days
Longer course for meningitis, pneumonia, osteomyelitis
Adjust antibiotics based on sensitivity
TORCH Infection Diagnosis
For suspected congenital infections, specific diagnostic tests should be performed:
| Infection | Diagnostic Tests |
|---|---|
| Toxoplasmosis | Toxoplasma-specific IgM and IgG antibodies, PCR of amniotic fluid or neonate’s blood |
| Rubella | Rubella-specific IgM antibodies, viral isolation from throat, urine, CSF |
| Cytomegalovirus | Viral culture or PCR of urine, saliva, or CSF within 2-3 weeks of birth |
| Herpes Simplex | PCR of vesicle fluid, CSF, blood; viral culture of lesions, oropharynx, conjunctiva |
| Syphilis | VDRL, RPR, FTA-ABS tests; dark-field microscopy of lesions |
| HIV | HIV DNA PCR (preferred for infants), HIV antibody test (may reflect maternal antibodies) |
Nursing Responsibilities in Diagnosis:
- Recognize and report subtle signs of infection promptly
- Obtain appropriate cultures before antibiotic administration, when possible
- Ensure adequate volume of blood for cultures (minimum 1 mL, ideally 2 mL)
- Properly label and transport specimens
- Document timing of sample collection and antibiotic administration
- Monitor for changes in clinical status
- Support parents during the diagnostic process
6. Treatment Strategies
The management of neonatal infections requires a multidisciplinary approach with prompt initiation of appropriate antimicrobial therapy and supportive care.
Empiric Antimicrobial Therapy
Empiric antibiotic therapy should be initiated as soon as cultures are obtained in neonates with suspected infection. The choice of antibiotics depends on the timing of presentation, local pathogen prevalence, and antimicrobial resistance patterns.
| Type of Infection | First-line Empiric Therapy | Alternative Regimens | Considerations |
|---|---|---|---|
| Early-Onset Sepsis | Ampicillin + Gentamicin | Ampicillin + Cefotaxime | Covers GBS, E. coli, Listeria; avoid routine cephalosporins due to resistance concerns |
| Late-Onset Sepsis | Vancomycin + Gentamicin or Cefotaxime | Vancomycin + Cefepime or Meropenem | Covers staphylococci (including CONS) and gram-negative organisms |
| Meningitis | Ampicillin + Cefotaxime or Gentamicin | Meropenem ± Vancomycin | Higher doses; consider adding vancomycin if staphylococcal meningitis suspected |
| Healthcare-Associated | Vancomycin + Piperacillin-Tazobactam | Vancomycin + Meropenem | Consider local antibiogram; add fluconazole/amphotericin B if fungal etiology suspected |
Pathogen-Specific Therapy
Bacterial Infections
- Group B Streptococcus: Penicillin G or Ampicillin for 10-14 days; 14-21 days for meningitis
- E. coli: Cefotaxime or Gentamicin for 10-14 days; 21+ days for meningitis
- Listeria monocytogenes: Ampicillin + Gentamicin for 14-21 days
- Staphylococcus aureus: Nafcillin/Oxacillin for MSSA; Vancomycin for MRSA
- CONS: Vancomycin for 7-10 days
Viral Infections
- HSV: Acyclovir 60 mg/kg/day divided q8h for 14-21 days
- CMV: Ganciclovir 12 mg/kg/day divided q12h for 6 weeks
- Enterovirus: Supportive care; IVIG may be considered
- HIV: Combination antiretroviral therapy based on current guidelines
Fungal Infections
- Candida species: Amphotericin B 1 mg/kg/day or Fluconazole 12 mg/kg/day
- Systemic candidiasis: Add Flucytosine if CNS involvement
- Duration: 14-21 days after last positive culture
TORCH Infection Treatment
- Toxoplasmosis: Pyrimethamine + Sulfadiazine + Leucovorin for 12 months
- Rubella: Supportive care; no specific therapy
- Syphilis: Penicillin G for 10-14 days
- Varicella: Acyclovir if severe disease
Supportive Care
Nursing Care for Neonates with Infections:
- Continuous Monitoring: Vital signs, respiratory effort, perfusion, urine output
- Maintenance of Thermoregulation: Neutral thermal environment
- Fluid and Electrolyte Management: Careful fluid administration, regular electrolyte checks
- Nutrition Support: Early enteral feeding when possible, TPN if needed
- Infection Control: Proper hand hygiene, isolation precautions as needed
- Medication Administration: Accurate dosing, appropriate dilution, monitoring for adverse effects
- Developmental Care: Minimal handling, clustered care, pain management
- Family Support: Education, emotional support, involvement in care
Duration of Therapy
| Clinical Scenario | Recommended Duration |
|---|---|
| Suspected sepsis with negative cultures and clinical improvement | 48-72 hours |
| Culture-proven bacteremia without focus | 7-10 days |
| Meningitis – Group B Streptococcus | 14-21 days |
| Meningitis – Gram-negative organisms | 21+ days |
| Soft tissue/bone/joint infections | 14-28 days |
| Endocarditis | 4-6 weeks |
| HSV infection – skin/mucous membranes only | 14 days |
| HSV infection – CNS or disseminated | 21 days |
Monitoring During Treatment:
- Serial CBC, CRP, procalcitonin to assess response to therapy
- Repeat blood cultures after 24-48 hours of therapy for persistent bacteremia
- Repeat CSF analysis after 24-48 hours for meningitis (controversial)
- Therapeutic drug monitoring for aminoglycosides and vancomycin
- Monitoring for adverse effects of antimicrobials (nephrotoxicity, ototoxicity, etc.)
When to Consider Stopping Antibiotics Early:
- Negative cultures at 36-48 hours
- No clinical signs of sepsis
- Normal or improved inflammatory markers
- Alternative diagnosis established
- Low pre-test probability of infection (started as precaution)
Prolonged unnecessary antibiotic exposure is associated with adverse effects including altered microbiome, antimicrobial resistance, invasive candidiasis, and necrotizing enterocolitis.
7. Prevention Strategies
Prevention of neonatal infections involves a multifaceted approach targeting maternal, perinatal, and postnatal interventions.
Prenatal/Maternal Interventions
- Maternal vaccinations (rubella, influenza, pertussis, etc.)
- Routine screening for GBS at 35-37 weeks gestation
- Treatment of maternal infections during pregnancy
- TORCH screening for high-risk pregnancies
- Adequate prenatal care and nutrition
- HIV antiretroviral therapy during pregnancy
Intrapartum Interventions
- Intrapartum antibiotic prophylaxis (IAP) for GBS colonized mothers
- Prompt treatment of chorioamnionitis
- Appropriate antiseptic techniques during delivery
- Limiting internal monitoring and procedures during labor
- Cesarean delivery for active genital herpes lesions
- Minimizing duration of rupture of membranes
Immediate Postnatal Care
- Proper cord care (chlorhexidine in resource-limited settings)
- Eye prophylaxis for gonorrheal and chlamydial infections
- Early initiation of breastfeeding
- Skin-to-skin contact with mother
- Hepatitis B vaccination and HBIG within 12 hours of birth
- Hand hygiene before handling the newborn
NICU Infection Control
- Strict hand hygiene protocols
- Aseptic techniques for all procedures
- Bundle care approaches for central lines (CLABSI prevention)
- Minimizing invasive procedures
- Optimizing nutrition (breast milk preferred)
- Judicious use of antibiotics
- Adequate staffing ratios
Prevention of Early-Onset GBS Disease
GBS Prevention Algorithm
Maternal GBS Screening at 35-37 weeks
Positive GBS Culture
Provide IAP during labor
Penicillin G (preferred) or Ampicillin
Alternative for PCN allergy: Cefazolin, Clindamycin, or Vancomycin
Negative GBS Culture
IAP not indicated except for:
– GBS bacteriuria during pregnancy
– Previous infant with GBS disease
– Unknown status with risk factors
Prevention of Healthcare-Associated Infections
Mnemonic: “HANDS CLEAN”
Central Line-Associated Bloodstream Infection (CLABSI) Prevention
| Prevention Strategy | Key Components |
|---|---|
| Central Line Insertion Bundle |
|
| Central Line Maintenance Bundle |
|
Probiotics and Neonatal Infections
Emerging evidence suggests that probiotics may have a role in preventing necrotizing enterocolitis (NEC) and late-onset sepsis in preterm infants. Probiotics help establish a healthy gut microbiome, strengthen intestinal barrier function, and modulate immune responses.
Potential Benefits of Probiotics:
- Reduced incidence of NEC (by approximately 30-50%)
- Possible reduction in late-onset sepsis
- Improved feeding tolerance
- Decreased time to full enteral feeds
Note: While evidence supports probiotic use, concerns about product standardization, optimal strain selection, and safety remain. Institutional protocols vary.
Nursing Role in Infection Prevention:
- Serving as hand hygiene champions
- Ensuring proper implementation of infection prevention bundles
- Educating families on infection prevention practices
- Monitoring for early signs of infection
- Participating in surveillance and quality improvement initiatives
- Advocating for appropriate antibiotic use and timely discontinuation
- Supporting breast milk feeding
8. Key Takeaways
Understanding Neonatal Infections
- Neonatal infections can be early-onset (0-72h) or late-onset (4-28d)
- TORCH infections are a special group of congenital infections
- Neonates are vulnerable due to immature immune systems
- Risk factors include prematurity, PROM, maternal infections, and invasive procedures
Clinical Presentation
- Signs and symptoms are often nonspecific
- Subtle changes in vital signs or behavior may be the first indicators
- Temperature instability, feeding intolerance, and respiratory distress are common
- Different pathogens may cause characteristic presentations
- A high index of suspicion is needed for early diagnosis
Diagnostic Approach
- Blood cultures remain the gold standard for diagnosis
- Complete septic workup includes CBC, CRP, blood cultures
- Lumbar puncture is crucial if meningitis is suspected
- Consider additional cultures (urine, tracheal) and imaging based on presentation
- Biomarkers like procalcitonin may aid in early diagnosis
Treatment Strategies
- Prompt initiation of empiric antibiotics is crucial
- Ampicillin + Gentamicin is standard for early-onset sepsis
- Tailor therapy based on culture results and clinical response
- Supportive care includes respiratory, cardiovascular, and nutritional support
- Consider antibiotic discontinuation at 36-48 hours if cultures are negative
Prevention Strategies
- Hand hygiene is the most important preventive measure
- Maternal GBS screening and IAP prevent early-onset GBS disease
- Implementation of care bundles reduces healthcare-associated infections
- Breastfeeding provides protective antibodies and probiotics
- Judicious use of antibiotics prevents resistance
Nursing Implications
- Early recognition of subtle signs of infection
- Proper specimen collection before antibiotics when possible
- Meticulous medication administration
- Implementation of infection prevention practices
- Family education and support
- Advocacy for appropriate antimicrobial stewardship
Essential Clinical Pearls:
- When in doubt, start antibiotics – neonatal sepsis can progress rapidly
- Always obtain cultures before starting antibiotics when possible
- Be vigilant for subtle signs – a change in behavior may be the first sign of infection
- Reassess the need for antibiotics at 36-48 hours
- Consider stopping antibiotics if cultures are negative, inflammatory markers are normal/improving, and there is an alternative explanation for clinical findings
- Prevention is more effective than treatment – strict adherence to hand hygiene and infection control practices is essential
- Support breastfeeding as it provides protective antibodies and probiotics
- Involve families in infection prevention education
