Neonatal Respiratory Distress Syndrome (RDS): Nursing Management
Comprehensive Nursing Guide for Caring for Neonates with RDS
Table of Contents
1. Introduction
Neonatal Respiratory Distress Syndrome (RDS), formerly known as Hyaline Membrane Disease, is a common respiratory disorder primarily affecting premature infants. It is characterized by insufficient production of pulmonary surfactant, leading to decreased lung compliance, alveolar collapse, and subsequent respiratory distress.
RDS occurs in approximately 24,000 infants born in the United States annually and is the most common cause of respiratory distress in premature infants. The incidence and severity of RDS are inversely proportional to gestational age, with nearly 98% of babies born at 24 weeks gestation developing RDS, dropping to just 5% by 34 weeks gestation, and less than 1% at term.
Key Risk Factors for RDS
- Prematurity (especially before 34 weeks gestation)
- Low birth weight
- Male gender
- Maternal diabetes
- Cesarean delivery without labor
- White race
- Perinatal asphyxia
- Maternal history of previous infant with RDS
- Multiple gestation (twins, triplets)
Nurses play a critical role in the early identification, management, and ongoing care of neonates with RDS. Understanding the pathophysiology, clinical presentation, and evidence-based interventions is essential for providing optimal nursing care to these vulnerable infants.
Back to Contents2. Pathophysiology
2.1 Fetal Lung Development
Understanding fetal lung development is crucial to comprehending the pathophysiology of RDS. Lung development occurs in five sequential stages:
| Stage | Gestational Age | Key Developments |
|---|---|---|
| Embryonic | 3-7 weeks | Formation of lung bud, mainstem bronchi, and pulmonary vessels |
| Pseudoglandular | 5-16 weeks | Development of bronchial tree, cartilage, and mucous glands |
| Canalicular | 16-25 weeks | Formation of respiratory bronchioles, alveolar ducts, beginning of type II cell differentiation and surfactant production |
| Saccular | 24-32 weeks | Development of terminal saccules, thinning of interstitium, increased surfactant production |
| Alveolar | 32 weeks – 2 years postnatal | Alveolar formation and maturation, increased surface area for gas exchange |
Premature birth interrupts this developmental sequence, resulting in structurally and functionally immature lungs. The degree of immaturity depends on the gestational age at birth.
2.2 Role of Surfactant
Pulmonary surfactant is a complex mixture of phospholipids (primarily dipalmitoylphosphatidylcholine) and specialized proteins produced by type II pneumocytes in the alveolar lining. Surfactant production begins around 20-22 weeks gestation but does not reach adequate levels until 34-36 weeks.
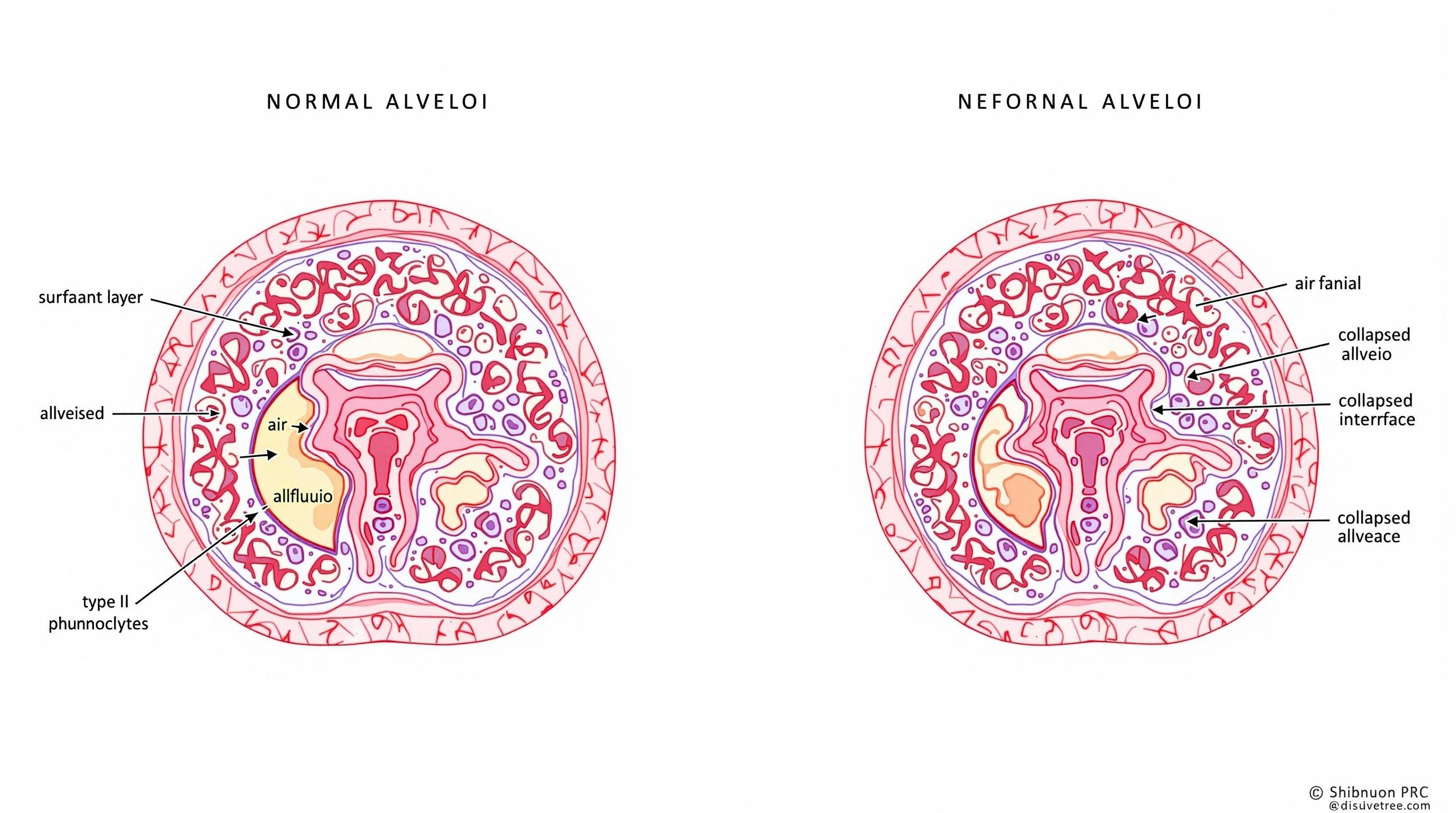
Figure 1: Comparison of normal alveoli (left) with surfactant lining versus alveoli in RDS (right) showing collapsed alveoli due to surfactant deficiency.
Surfactant performs several critical functions:
- Reduces surface tension at the air-liquid interface within alveoli
- Prevents alveolar collapse at end-expiration
- Reduces the work of breathing by increasing lung compliance
- Maintains alveolar stability by preventing fluid accumulation in the alveolar space
- Contributes to host defense against pulmonary infections
Surfactant contains four specific proteins (SP-A, SP-B, SP-C, and SP-D) that are essential for its function:
- SP-A and SP-D: Immunoregulatory functions, pathogen clearance
- SP-B: Critical for surfactant spreading and stability
- SP-C: Works with SP-B to maintain surfactant function
2.3 Pathogenesis of RDS
The primary defect in RDS is insufficient surfactant production due to lung immaturity. This deficiency leads to a cascade of physiological disturbances:
- Increased surface tension: With insufficient surfactant, alveolar surface tension increases dramatically
- Alveolar collapse: High surface tension promotes alveolar collapse (atelectasis), reducing the functional respiratory surface area
- Decreased lung compliance: The lungs become stiff and non-compliant, increasing the work of breathing
- Ventilation-perfusion mismatch: Perfusion of non-ventilated alveoli leads to right-to-left shunting
- Respiratory failure: Progressive hypoxemia and hypercapnia develop
- Secondary injury: Damage to the respiratory epithelium triggers inflammation and pulmonary edema, further inactivating surfactant
The pathological process becomes cyclical, as surfactant-deficient collapsed alveoli lead to epithelial damage, which reduces surfactant production and increases inflammatory processes, further exacerbating the condition.
3. Clinical Presentation
3.1 Symptoms and Signs
RDS typically presents within minutes to hours after birth, with symptoms becoming evident immediately after delivery in severe cases. The clinical presentation includes:
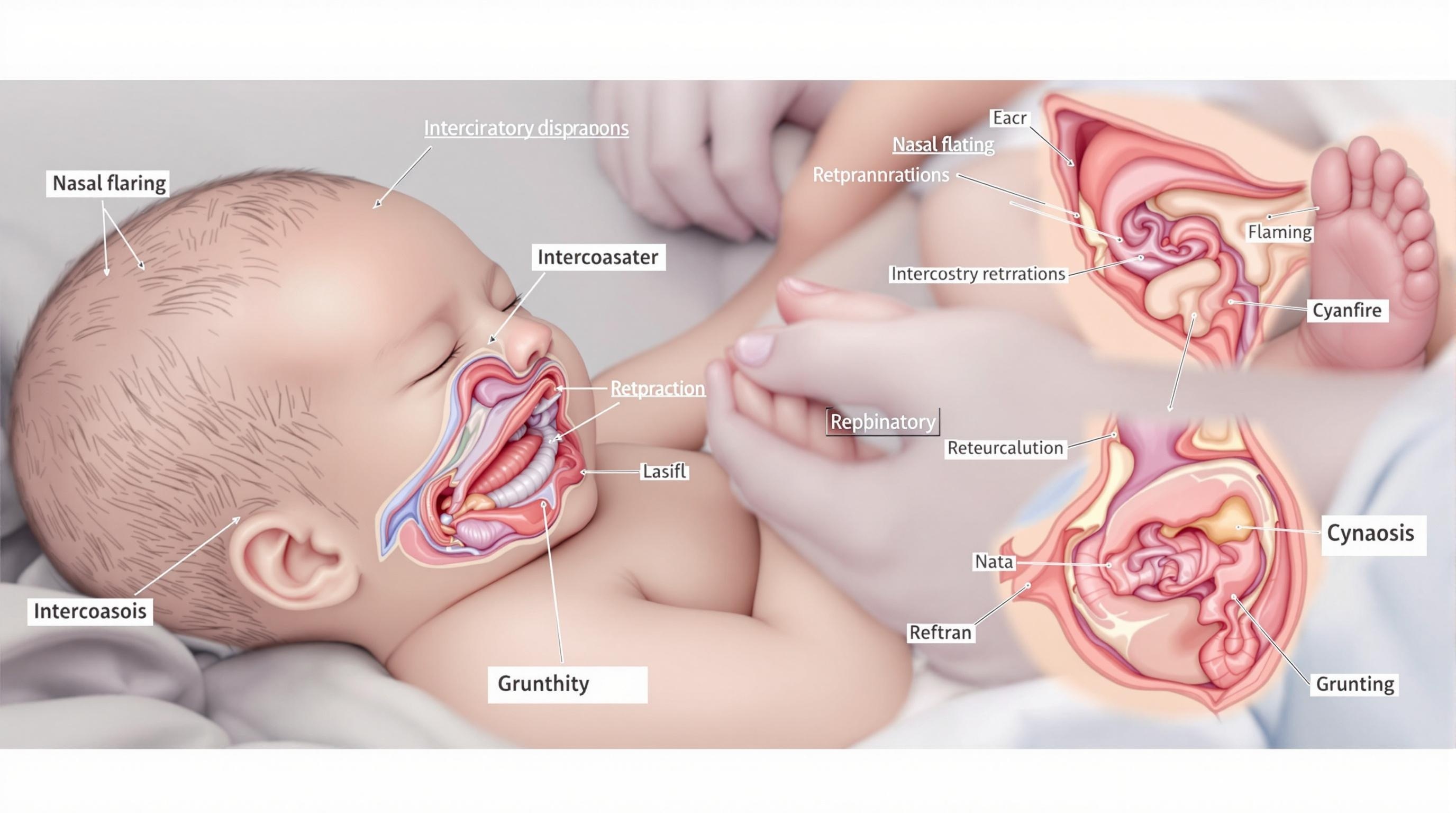
Figure 2: Clinical signs of respiratory distress syndrome in a neonate, showing key assessment findings.
Key Clinical Features of RDS
- Tachypnea: Respiratory rate >60 breaths per minute
- Expiratory grunting: Attempt to maintain positive end-expiratory pressure
- Nasal flaring: Compensatory mechanism to decrease airway resistance
- Retractions: Subcostal, intercostal, suprasternal – visible chest wall indrawing during inspiration
- Cyanosis: Central cyanosis that responds to supplemental oxygen
- Diminished breath sounds: Often generalized and bilateral
- Poor peripheral perfusion: Pallor, delayed capillary refill
- Lethargy or decreased activity: Especially as condition worsens
- Apneic episodes: More common as RDS progresses
Without treatment, symptoms progressively worsen over the first 48-72 hours. The infant becomes increasingly tachypneic and develops more prominent retractions. Respiratory effort may eventually decrease as the infant tires, leading to apnea, respiratory failure, and cardiopulmonary arrest.
3.2 Clinical Assessment Tool
The “SURFACTANT” Mnemonic for RDS Assessment
S – Skin color: Observe for central cyanosis or pallor
U – Use of accessory muscles: Check for retractions (subcostal, intercostal, suprasternal)
R – Respiratory rate: Count for a full minute (tachypnea >60 breaths/min)
F – Flaring of nostrils: Observe during respiratory cycle
A – Auscultation: Listen for decreased breath sounds, crackles
C – Cry and activity: Assess quality of cry and level of activity
T – Tone: Evaluate muscle tone, which may be decreased in severe distress
A – Air hunger: Look for signs of increased work of breathing
N – Nail bed perfusion: Check capillary refill time
T – Temperature: Monitor for temperature instability
The Silverman-Anderson score is also commonly used to assess the severity of respiratory distress in neonates. It evaluates five parameters: chest movement, intercostal retractions, xiphoid retractions, nares dilation, and expiratory grunting. Each parameter is scored from 0-2, with a maximum score of 10 indicating severe respiratory distress.
| Score | Severity of RDS | Clinical Implications |
|---|---|---|
| 0-3 | Mild | Close monitoring, may need minimal respiratory support |
| 4-6 | Moderate | Requires respiratory support, consider surfactant therapy |
| 7-10 | Severe | Immediate intervention, intubation and surfactant therapy indicated |
4. Diagnosis
4.1 Diagnostic Criteria
The diagnosis of RDS is based on a combination of clinical presentation, radiographic findings, and laboratory results. Key diagnostic criteria include:
- Prematurity: Gestational age less than 37 weeks
- Clinical signs of respiratory distress beginning shortly after birth
- Characteristic chest radiograph findings: Ground-glass appearance, air bronchograms, decreased lung volumes
- Increasing oxygen requirement to maintain normal blood gas values
- Exclusion of other causes of neonatal respiratory distress
4.2 Differential Diagnosis
Several conditions can mimic the clinical presentation of RDS in neonates:
| Condition | Key Distinguishing Features | Radiographic Findings |
|---|---|---|
| Transient Tachypnea of the Newborn (TTN) | Term or late preterm infants, rapid improvement within 24-48 hours, less severe hypoxemia | Perihilar streaking, fluid in fissures, hyperinflation, rapid clearing |
| Neonatal Pneumonia | Maternal risk factors (chorioamnionitis, prolonged rupture of membranes), fever, elevated inflammatory markers | Patchy infiltrates, pleural effusion, may resemble RDS |
| Meconium Aspiration Syndrome | Term or post-term infants, meconium-stained amniotic fluid, severe hypoxemia, early onset | Patchy, coarse infiltrates, hyperinflation, areas of atelectasis |
| Pneumothorax | Sudden deterioration, asymmetrical chest movement, shifted heart sounds | Unilateral hyperlucency, mediastinal shift, visible pleural line |
| Congenital Heart Disease | Heart murmur, differential cyanosis (PDA), persistent hypoxemia despite oxygen | Cardiomegaly, increased pulmonary vascular markings, normal lung fields |
| Persistent Pulmonary Hypertension of the Newborn (PPHN) | Term or post-term infant, severe hypoxemia refractory to oxygen, labile oxygenation | Clear lung fields or minimal changes, enlarged cardiac silhouette |
4.3 Diagnostic Tests
Several diagnostic tests and procedures aid in diagnosing RDS and assessing its severity:
Key Diagnostic Tests for RDS
- Chest Radiography: Classic findings include diffuse reticulogranular pattern (“ground glass” appearance), air bronchograms, and low lung volumes
- Arterial Blood Gas (ABG) Analysis: Shows hypoxemia, hypercapnia, and respiratory or mixed acidosis
- Pulse Oximetry: Continuous monitoring of oxygen saturation
- Complete Blood Count (CBC): To rule out infection/sepsis
- Blood Cultures: If infection is suspected
- Echocardiography: To evaluate for structural heart disease and assess pulmonary hypertension
- Lung Ultrasound: Increasingly used for RDS diagnosis, shows coalescent B-lines and pleural line abnormalities
The lamellar body count in amniotic fluid can be used to assess fetal lung maturity before birth, with counts less than 15,000/μL suggesting immature lungs and increased risk for RDS.
5. Nursing Management
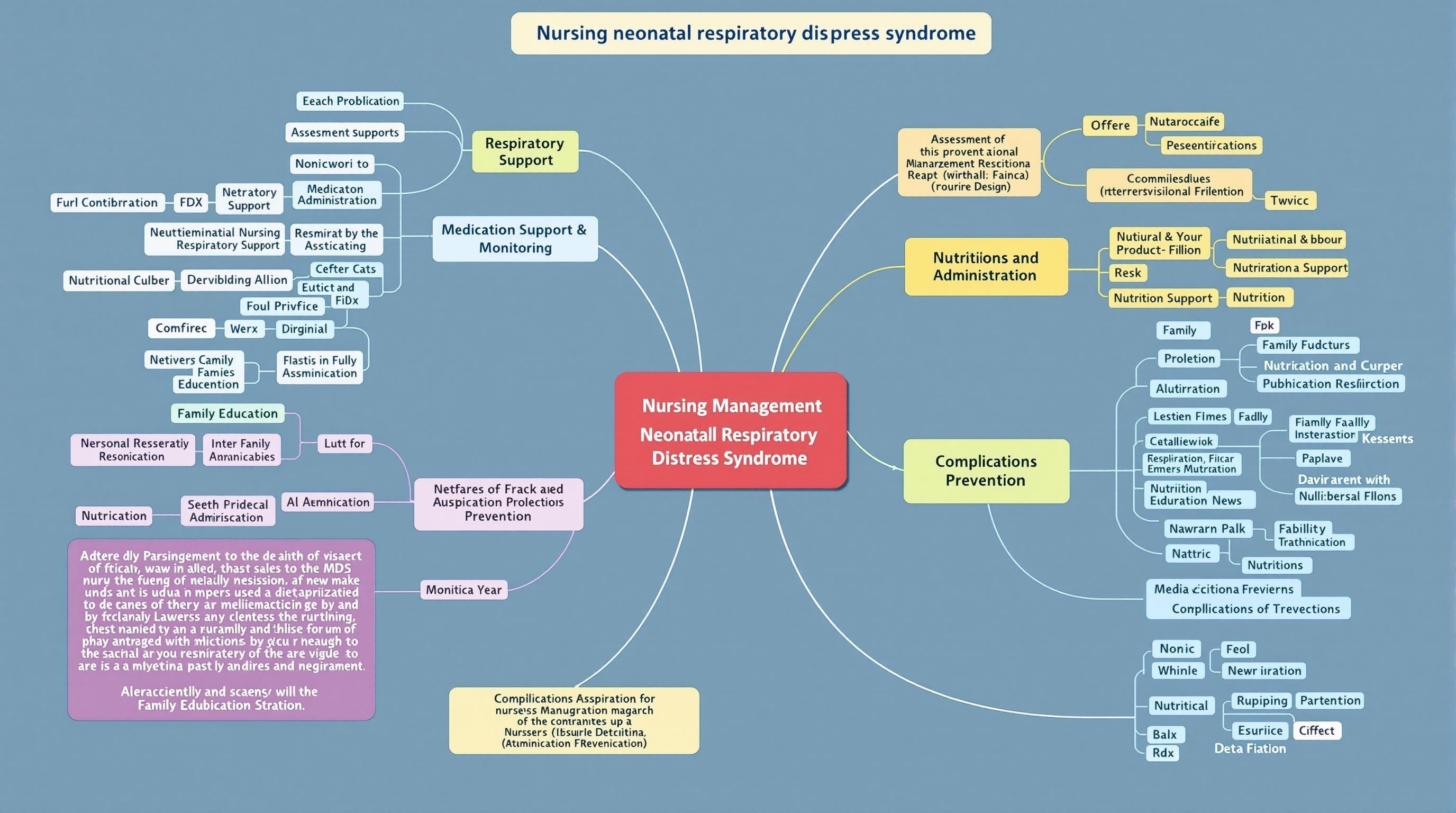
Figure 3: Mind map of comprehensive nursing management for neonates with respiratory distress syndrome.
5.1 Assessment
Comprehensive and ongoing assessment is the foundation of effective nursing care for neonates with RDS:
- Initial Assessment:
- Complete head-to-toe assessment with focus on respiratory status
- Document respiratory rate, pattern, work of breathing
- Assess for retractions, nasal flaring, grunting, cyanosis
- Evaluate breath sounds in all lung fields
- Note oxygen requirements and response to oxygen therapy
- Ongoing Monitoring:
- Perform respiratory assessments every 1-2 hours or more frequently as indicated
- Monitor for signs of deterioration or improvement
- Assess work of breathing, respiratory rate, and effort
- Document response to interventions and therapies
- Vital Signs:
- Monitor heart rate, respiratory rate, blood pressure, and temperature
- Observe for signs of compensation (tachycardia) or decompensation (bradycardia)
- Oxygenation Assessment:
- Maintain continuous pulse oximetry
- Target oxygen saturation per unit protocol (typically 90-95%)
- Monitor pre-ductal and post-ductal saturations if PPHN is suspected
5.2 Respiratory Support
Respiratory support is the cornerstone of RDS management, with interventions tailored to the severity of the condition:
Continuous Positive Airway Pressure (CPAP)
CPAP is the initial respiratory support for many infants with RDS:
- Maintains alveolar recruitment and prevents collapse
- Decreases work of breathing
- Improves oxygenation by increasing functional residual capacity
- Nursing considerations:
- Ensure proper positioning of nasal prongs or mask
- Monitor for pressure injuries to nares or face
- Assess for abdominal distension (CPAP belly)
- Document CPAP settings (pressure, FiO2) and adjustments
Surfactant Replacement Therapy
Exogenous surfactant administration is a key intervention for moderate to severe RDS:
- Assists with preparation and administration of surfactant therapy
- Monitors infant’s tolerance during administration
- Assesses response to therapy (oxygenation, ventilation, work of breathing)
- Observes for complications: pulmonary hemorrhage, airway obstruction, transient bradycardia, desaturation
- Prepares for less invasive surfactant administration (LISA) techniques when applicable
Mechanical Ventilation
For infants with severe RDS or those who fail CPAP therapy:
- Assists with intubation procedure
- Secures endotracheal tube and documents position
- Monitors ventilator settings and alarms
- Maintains patent airway through appropriate suctioning techniques
- Implements ventilator bundle care to prevent complications
- Documents ventilator changes and infant’s response
Oxygen Therapy
- Administers supplemental oxygen as prescribed
- Monitors FiO2 requirements
- Adjusts oxygen delivery based on saturation targets
- Documents oxygen weaning attempts and infant tolerance
5.3 Monitoring
Continuous and comprehensive monitoring is essential for neonates with RDS:
- Cardiorespiratory Monitoring:
- Continuous heart rate, respiratory rate, and oxygen saturation monitoring
- Set appropriate alarm limits and ensure alarms are audible
- Document parameters hourly or more frequently as needed
- Blood Gas Monitoring:
- Assist with obtaining arterial, venous, or capillary blood samples
- Interpret blood gas results and report abnormal findings
- Monitor trends in pH, pCO2, pO2, and base deficit
- Fluid Balance:
- Monitor intake and output strictly
- Assess for signs of fluid overload or dehydration
- Weigh infant daily at the same time with the same scale
- Hemodynamic Monitoring:
- Monitor blood pressure and mean arterial pressure
- Assess perfusion (capillary refill, skin color, peripheral pulses)
- Evaluate for signs of shock or cardiovascular compromise
5.4 Medication Administration
Various medications are used in the management of RDS:
| Medication | Purpose | Nursing Considerations |
|---|---|---|
| Surfactant (Beractant, Poractant alfa, Calfactant) | Replace deficient natural surfactant | Administer via endotracheal tube; monitor for desaturation, bradycardia during administration; position infant properly during and after administration |
| Caffeine Citrate | Stimulate respiratory drive, prevent apnea, facilitate extubation | Monitor for tachycardia, jitteriness, feeding intolerance; administer loading dose slowly |
| Antibiotics | Treat suspected or confirmed infection | Obtain cultures before starting therapy; administer on schedule; monitor for adverse effects |
| Vitamin A | Reduce risk of bronchopulmonary dysplasia | Administer intramuscularly; rotate injection sites; document administration |
| Diuretics | Manage pulmonary edema | Monitor electrolytes, urine output; observe for dehydration |
| Inotropes (Dopamine, Dobutamine) | Support blood pressure and cardiac output | Administer via central line; monitor blood pressure frequently; titrate according to response |
5.5 Nutritional Support
Maintaining adequate nutrition is challenging but essential for infants with RDS:
- Parenteral Nutrition:
- Assist with placement and maintenance of central venous access
- Monitor administration of parenteral nutrition and lipids
- Document intake and assess glucose levels
- Monitor for complications (line infection, metabolic disturbances)
- Enteral Nutrition:
- Assess readiness for enteral feeding
- Measure gastric residuals before feeds
- Implement minimal enteral nutrition protocols when appropriate
- Monitor tolerance to feedings (residuals, abdominal distension, emesis)
- Support and facilitate breastfeeding or breast milk feeding when possible
- Growth Monitoring:
- Track weight, length, and head circumference
- Plot measurements on appropriate growth charts
- Calculate daily weight gain and report inadequate growth
5.6 Thermoregulation
Maintaining a neutral thermal environment is critical for neonates with RDS:
- Temperature Monitoring:
- Monitor axillary temperature every 3-4 hours or continuously with skin probe
- Maintain axillary temperature between 36.5°C and 37.5°C
- Document temperature readings and interventions
- Environmental Control:
- Adjust incubator or radiant warmer settings to maintain neutral thermal environment
- Minimize heat loss during procedures
- Warm all gases (oxygen, air) delivered to the infant
- Pre-warm all items that come in contact with the infant
- Prevention of Heat Loss:
- Use plastic wrap or bags for extremely preterm infants
- Minimize opening of incubator doors
- Use appropriate insulation (hats, blankets) during transport
- Warm fluids and blood products before administration
5.7 Positioning and Handling
Proper positioning can optimize respiratory function and comfort:
- Optimal Positioning:
- Position in slight head elevation (30°) to decrease work of breathing
- Use prone position when clinically stable to improve oxygenation and lung mechanics
- Implement quarter turns every 2-4 hours to prevent pressure injuries
- Maintain head in midline position to prevent uneven ventilation
- Developmental Care:
- Implement boundaries using positioning aids or rolled blankets
- Promote flexed positioning similar to in-utero position
- Support physiological flexion of extremities
- Cluster care activities to minimize handling and disruptions
- Minimal Handling:
- Limit interventions to essential care
- Coordinate care activities to provide rest periods
- Monitor physiological responses to handling and adjust care accordingly
- Implement “time-out” if infant shows signs of stress during procedures
6. Prevention
Prevention strategies for RDS focus on enhancing fetal lung maturation and optimizing perinatal care:
Antenatal Corticosteroids
The most effective preventive intervention for RDS:
- Administered to mothers at risk of preterm delivery between 23-34 weeks gestation
- Standard regimen: Betamethasone 12 mg IM, two doses 24 hours apart, or Dexamethasone 6 mg IM, four doses 12 hours apart
- Accelerates fetal lung maturation and surfactant production
- Reduces RDS incidence by 40-60%
- Most effective when given 24 hours to 7 days before delivery
- Additional benefits: reduced risk of intraventricular hemorrhage, necrotizing enterocolitis, and neonatal mortality
Prevention of Preterm Birth
- Early identification and management of pregnant women at risk for preterm delivery
- Tocolytic therapy to delay delivery when appropriate
- Magnesium sulfate administration for neuroprotection
- Treatment of maternal infections and medical conditions that may trigger preterm labor
- Cervical cerclage for women with cervical insufficiency
- Progesterone therapy for women with history of preterm birth or short cervix
Optimal Delivery Planning
- Delivery at a center with appropriate neonatal intensive care facilities
- In-utero transfer when delivery in a facility without advanced neonatal care is anticipated
- Presence of skilled neonatal resuscitation team at delivery
- Delayed cord clamping when appropriate to improve hemodynamic transition
- Avoid unnecessary cesarean sections, particularly elective procedures before 39 weeks
Prophylactic Surfactant
- Previously, prophylactic surfactant was given to extremely preterm infants (< 28 weeks)
- Current evidence favors early CPAP with selective surfactant administration
- Prophylactic surfactant may still be considered for extremely preterm infants who require intubation in the delivery room
7. Complications
Despite advances in management, RDS can lead to several short-term and long-term complications:
| Complication | Description | Nursing Implications |
|---|---|---|
| Bronchopulmonary Dysplasia (BPD) | Chronic lung disease characterized by oxygen dependency beyond 36 weeks corrected gestational age | Monitor respiratory status, implement lung-protective ventilation strategies, minimize oxygen exposure, administer medications as prescribed, educate parents about long-term care |
| Air Leak Syndromes | Pneumothorax, pneumomediastinum, pulmonary interstitial emphysema due to alveolar rupture | Monitor for sudden deterioration, asymmetrical chest movement, decreased breath sounds; assist with chest tube placement and management if needed |
| Patent Ductus Arteriosus (PDA) | Persistent opening between pulmonary artery and aorta, increasing pulmonary blood flow | Monitor for heart murmur, widened pulse pressure, decreased peripheral perfusion; administer medications as ordered; prepare for surgical ligation if indicated |
| Intraventricular Hemorrhage (IVH) | Bleeding into the ventricles of the brain, more common in preterm infants with RDS | Monitor neurological status, head circumference; maintain stable blood pressure; minimize handling during acute phase; support parents during diagnosis |
| Retinopathy of Prematurity (ROP) | Abnormal retinal vascular development associated with oxygen therapy | Maintain oxygen saturations within prescribed range; assist with eye examinations; educate parents about follow-up care |
| Necrotizing Enterocolitis (NEC) | Inflammatory bowel disease affecting primarily premature infants | Monitor abdominal distension, residuals, stool for blood; implement feeding protocols; assist with abdominal X-rays; administer antibiotics as prescribed |
| Sepsis | Systemic infection, risk increased due to invasive procedures and immature immune system | Monitor for signs of infection; assist with obtaining cultures; administer antibiotics as ordered; implement infection prevention practices |
| Neurodevelopmental Impairment | Long-term neurological sequelae, including cerebral palsy, cognitive delays, sensory impairments | Support early intervention services; educate parents about developmental follow-up; implement developmental care practices |
8. Family Education
Family education and support are integral components of nursing care for infants with RDS:
Education About RDS
- Explain RDS pathophysiology in simple, understandable terms
- Discuss causes, risk factors, and typical course of the condition
- Review diagnostic tests and their purposes
- Explain treatment modalities and expected outcomes
- Provide written materials and reliable online resources
NICU Orientation
- Orient parents to the NICU environment and equipment
- Explain the purpose of monitors, alarms, and respiratory support devices
- Review NICU policies regarding visitation, hand hygiene, and infection control
- Introduce the healthcare team members and their roles
- Provide information about support services available to families
Involvement in Care
- Encourage parental participation in care activities as appropriate
- Teach comfort measures like gentle touch, talking to infant, kangaroo care when stable
- Involve parents in feeding decisions and support breastfeeding if desired
- Provide opportunities for skin-to-skin contact when medically appropriate
- Encourage parents to personalize the infant’s space with family photos or mementos
Emotional Support
- Acknowledge parental feelings of grief, guilt, anxiety, and powerlessness
- Provide realistic but hopeful information about prognosis
- Connect families with social workers, chaplains, or parent support groups
- Encourage expression of emotions and concerns
- Recognize signs of parental stress or depression and refer for additional support
Discharge Planning and Follow-up
- Begin discharge planning early, addressing potential home care needs
- Teach parents about medication administration, equipment use if needed
- Provide CPR and emergency response training
- Review signs of respiratory distress that require medical attention
- Ensure understanding of follow-up appointments and developmental screening
- Connect with community resources and early intervention services
The “FAMILY” Approach to Parent Education
F – Foster a supportive environment for learning
A – Assess parents’ current understanding and needs
M – Modify education based on parents’ readiness to learn
I – Involve parents in care as much as possible
L – Listen to concerns and provide emotional support
Y – Yield detailed discharge instructions and follow-up plans
9. References
- Sweet, D. G., Carnielli, V., Greisen, G., Hallman, M., Ozek, E., Plavka, R., Saugstad, O. D., Simeoni, U., Speer, C. P., Vento, M., Visser, G. H., & Halliday, H. L. (2019). European Consensus Guidelines on the Management of Respiratory Distress Syndrome – 2019 Update. Neonatology, 115(4), 432-450.
- Hermansen, C. L., & Mahajan, A. (2015). Newborn Respiratory Distress. American Family Physician, 92(11), 994-1002.
- Polin, R. A., Carlo, W. A., & Committee on Fetus and Newborn. (2014). Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics, 133(1), 156-163.
- Roberts, D., Brown, J., Medley, N., & Dalziel, S. R. (2017). Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews, 3, CD004454.
- National Institute of Child Health and Human Development Neonatal Research Network. (2020). Respiratory outcomes of the surfactant positive pressure and oximetry randomized trial (SUPPORT). The Journal of Pediatrics, 141(2), 221-242.
- Gardner, S. L., Carter, B. S., Enzman-Hines, M., & Hernandez, J. A. (2021). Merenstein & Gardner’s Handbook of Neonatal Intensive Care (9th ed.). Elsevier.
- Davidson, J. E., Aslakson, R. A., Long, A. C., Puntillo, K. A., Kross, E. K., Hart, J., Cox, C. E., Wunsch, H., Wickline, M. A., Nunnally, M. E., Netzer, G., Kentish-Barnes, N., Sprung, C. L., Hartog, C. S., Coombs, M., Gerritsen, R. T., Hopkins, R. O., Franck, L. S., Skrobik, Y., Kon, A. A., … Curtis, J. R. (2017). Guidelines for Family-Centered Care in the Neonatal, Pediatric, and Adult ICU. Critical Care Medicine, 45(1), 103-128.
