Physiological Regulation of Fluid, Electrolyte & Acid-Base Balances
A Comprehensive Nursing Review
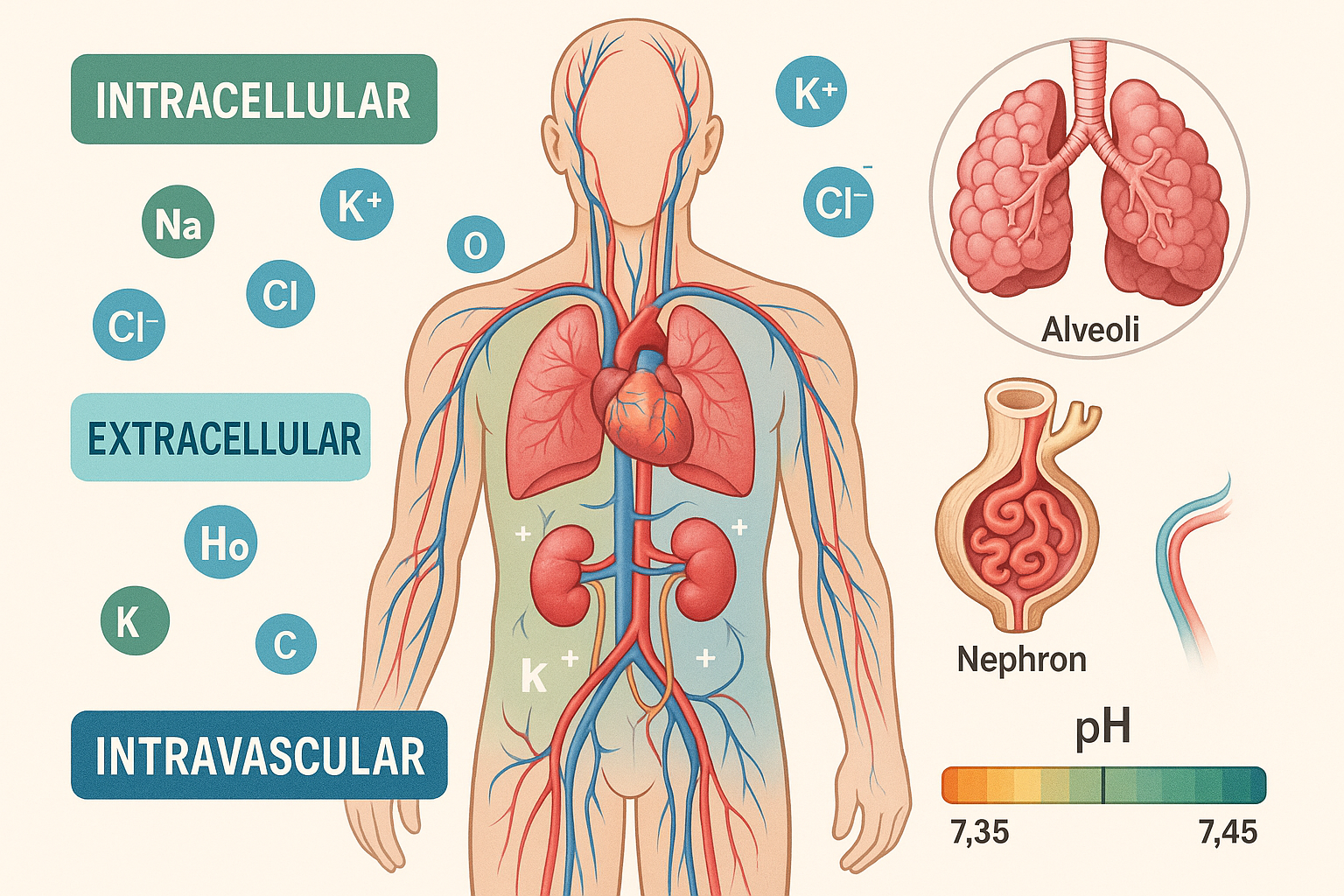
Table of Contents
Introduction to Fluid Balance
Key Concept
Fluid, electrolyte, and acid-base balance are fundamental physiological processes that maintain cellular function and overall homeostasis. Understanding these mechanisms is crucial for nursing practice as imbalances can lead to serious complications and require immediate intervention.
The human body consists of approximately 50-60% water by weight, with this percentage varying based on age, gender, and body composition. This fluid serves multiple critical functions including temperature regulation, nutrient transport, waste elimination, and maintenance of cellular integrity. The delicate balance of these fluids, along with dissolved electrolytes and pH levels, must be precisely maintained for optimal physiological function.
Nursing Tip
Always consider the patient’s baseline fluid status, comorbidities, and current medications when assessing fluid, electrolyte, and acid-base balance. Small changes can have significant physiological impacts, especially in vulnerable populations such as elderly patients, infants, and those with chronic diseases.
Physiological Importance
Cellular Function
Maintains cell membrane integrity and facilitates nutrient and waste exchange across cellular boundaries.
Hemodynamic Stability
Supports adequate blood volume and pressure for proper organ perfusion and cardiovascular function.
Metabolic Processes
Enables enzymatic reactions and maintains optimal pH for biochemical processes throughout the body.
Body Fluid Compartments
The body’s fluid is distributed among distinct compartments, each with specific functions and characteristics. Understanding these compartments is essential for recognizing fluid shifts and imbalances in clinical practice.
Intracellular Fluid (ICF)
Volume: ~28L (40% of body weight)
Primary electrolytes: K+, PO4³⁻, Mg²⁺
Function: Maintains cell volume and supports metabolic processes
The intracellular compartment contains the majority of body water and is separated from extracellular fluid by the cell membrane. This compartment is rich in potassium and phosphate ions, which are essential for cellular metabolism and protein synthesis.
Extracellular Fluid (ECF)
Volume: ~14L (20% of body weight)
Primary electrolytes: Na+, Cl⁻, HCO3⁻
Components: Plasma, interstitial fluid, transcellular fluid
Extracellular fluid surrounds cells and includes plasma within blood vessels and interstitial fluid in tissues. This compartment is primarily regulated by sodium levels and serves as the transport medium for nutrients, hormones, and waste products.
Mnemonic: Fluid Compartments
“ICF Keeps Potassium, ECF Saves Sodium”
- • Intracellular fluid = K+ (potassium)
- • Extracellular fluid = Sodium (Na+)
Fluid Movement Mechanisms
Osmosis
Water movement across semipermeable membranes from low to high solute concentration
Diffusion
Passive movement of solutes from high to low concentration gradients
Active Transport
Energy-dependent movement against concentration gradients (Na+/K+ pump)
Electrolyte Regulation
Electrolytes are charged particles that conduct electrical current in body fluids. Their precise regulation is crucial for nerve conduction, muscle contraction, fluid balance, and acid-base homeostasis.
| Electrolyte | Normal Range | Primary Functions | Regulation Site |
|---|---|---|---|
| Sodium (Na+) | 136-145 mEq/L | Fluid balance, nerve conduction, muscle contraction | Kidneys (aldosterone) |
| Potassium (K+) | 3.5-5.0 mEq/L | Cardiac rhythm, muscle contraction, acid-base balance | Kidneys (aldosterone) |
| Chloride (Cl⁻) | 98-107 mEq/L | Acid-base balance, osmotic pressure | Kidneys (follows sodium) |
| Bicarbonate (HCO3⁻) | 22-28 mEq/L | Primary buffer system, pH regulation | Kidneys, lungs |
| Calcium (Ca²+) | 8.5-10.5 mg/dL | Bone health, muscle contraction, blood clotting | Parathyroid hormone, vitamin D |
| Magnesium (Mg²+) | 1.3-2.1 mg/dL | Enzyme activation, protein synthesis, muscle function | Kidneys, intestines |
| Phosphate (PO4³⁻) | 3.0-4.5 mg/dL | Energy metabolism, bone mineralization, acid-base balance | Kidneys, parathyroid hormone |
Mnemonic: Major Electrolytes
“Some People Can’t Make Calcium Magnesium Perfect”
- • Sodium (Na+)
- • Potassium (K+)
- • Chloride (Cl⁻)
- • Magnesium (Mg²+)
- • Calcium (Ca²+)
- • Magnesium (Mg²+)
- • Phosphate (PO4³⁻)
Hormonal Regulation
Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) is the primary regulator of sodium and potassium balance:
- • Low blood volume/pressure triggers renin release
- • Angiotensin II stimulates aldosterone production
- • Aldosterone increases sodium retention and potassium excretion
- • Water follows sodium, increasing blood volume
Antidiuretic Hormone (ADH)
ADH regulates water balance by controlling water reabsorption in the kidneys:
- • Released when plasma osmolality increases
- • Increases water reabsorption in collecting ducts
- • Decreases urine output and concentrates urine
- • Helps maintain plasma osmolality at 280-290 mOsm/kg
Acid-Base Balance
Acid-base balance refers to the precise regulation of hydrogen ion concentration (pH) in body fluids. Normal blood pH must be maintained within the narrow range of 7.35-7.45 for optimal cellular function and enzyme activity.
pH Scale Understanding
Acidosis
pH < 7.35
Excess H+ ions
Normal
pH 7.35-7.45
Balanced state
Alkalosis
pH > 7.45
Excess OH⁻ ions
Buffer Systems
The body maintains acid-base balance through three primary buffer systems that work together to neutralize excess acids or bases:
1. Bicarbonate Buffer System
Most important system (75% of buffering)
H₂CO₃ ⇌ H⁺ + HCO₃⁻
Regulated by lungs (CO₂) and kidneys (HCO₃⁻)
2. Phosphate Buffer System
Important in ICF and urine
H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻
Most effective at physiological pH
3. Protein Buffer System
Hemoglobin and plasma proteins
Amino acids can accept or donate H⁺
Immediate response to pH changes
Mnemonic: Buffer Systems
“Big People Play”
- • Bicarbonate (most important)
- • Phosphate (ICF and urine)
- • Protein (immediate response)
Compensatory Mechanisms
Respiratory Compensation
Response time: Minutes to hours
- • Acidosis → Hyperventilation (blow off CO₂)
- • Alkalosis → Hypoventilation (retain CO₂)
- • Controlled by medullary respiratory centers
- • Can achieve partial compensation
Renal Compensation
Response time: Hours to days
- • Acidosis → Retain HCO₃⁻, excrete H⁺
- • Alkalosis → Excrete HCO₃⁻, retain H⁺
- • Most powerful compensatory mechanism
- • Can achieve complete compensation
Regulatory Mechanisms
The body employs multiple integrated systems to maintain fluid, electrolyte, and acid-base homeostasis. Understanding these mechanisms is crucial for recognizing pathological states and implementing appropriate interventions.
Renal Regulation
Nephron Function in Homeostasis
The functional unit of the kidney plays a central role in fluid and electrolyte balance:
Glomerular Filtration
- • Filters 180L of plasma daily
- • Pressure-dependent process
- • Autoregulated by myogenic and tubuloglomerular feedback
Tubular Reabsorption
- • Proximal tubule: 65% Na⁺, Cl⁻, water
- • Loop of Henle: concentrates urine
- • Distal tubule: fine-tunes electrolyte balance
Cardiovascular Regulation
Baroreceptor Response
Specialized pressure sensors in the cardiovascular system detect changes in blood volume and pressure:
↓ Blood Volume/Pressure
Baroreceptors Detect Change
Sympathetic Nervous System Activation
↑ Heart Rate, ↑ Contractility, Vasoconstriction
RAAS Activation → Fluid Retention
Endocrine Regulation
| Hormone | Source | Target | Effect |
|---|---|---|---|
| ADH (Vasopressin) | Posterior pituitary | Kidney collecting ducts | ↑ Water reabsorption |
| Aldosterone | Adrenal cortex | Kidney distal tubule | ↑ Na⁺ retention, ↑ K⁺ excretion |
| Parathyroid Hormone | Parathyroid glands | Bones, kidneys, intestines | ↑ Ca²⁺, ↓ PO₄³⁻ |
| Atrial Natriuretic Peptide | Cardiac atria | Kidneys, blood vessels | ↑ Na⁺ excretion, vasodilation |
Nursing Assessment
Comprehensive assessment of fluid, electrolyte, and acid-base status requires systematic evaluation of clinical signs, symptoms, and laboratory values. Early detection of imbalances is crucial for preventing complications.
Assessment Priority Framework
Use the ABCDE approach for systematic assessment:
- • Airway – Check for patency and protection
- • Breathing – Assess respiratory rate, depth, and effort
- • Circulation – Evaluate hemodynamic status
- • Disability – Neurological assessment
- • Exposure – Full body examination including skin
Physical Assessment Parameters
Fluid Status Indicators
Hypovolemia Signs:
- • Dry mucous membranes
- • Decreased skin turgor
- • Hypotension, tachycardia
- • Concentrated urine, oliguria
- • Altered mental status
Hypervolemia Signs:
- • Edema (pitting/non-pitting)
- • Jugular vein distension
- • Crackles, dyspnea
- • Weight gain
- • Hypertension
Electrolyte Imbalance Signs
Hyponatremia:
- • Confusion, altered LOC
- • Muscle cramps, weakness
- • Nausea, vomiting
- • Seizures (severe cases)
Hyperkalemia:
- • Cardiac arrhythmias
- • Muscle weakness/paralysis
- • Paresthesias
- • ECG changes (peaked T waves)
Mnemonic: Dehydration Assessment
“DAMP SKIN”
- • Dry mucous membranes
- • Altered mental status
- • Muscle weakness
- • Poor skin turgor
- • Sunken eyes
- • Kidney function decreased (oliguria)
- • Increased heart rate
- • Nausea and vomiting
Laboratory Assessment
Key Laboratory Values
Serum Electrolytes
- • Sodium (Na⁺): 136-145 mEq/L
- • Potassium (K⁺): 3.5-5.0 mEq/L
- • Chloride (Cl⁻): 98-107 mEq/L
- • Bicarbonate (HCO₃⁻): 22-28 mEq/L
Arterial Blood Gas
- • pH: 7.35-7.45
- • PCO₂: 35-45 mmHg
- • PO₂: 80-100 mmHg
- • HCO₃⁻: 22-28 mEq/L
- • Base excess: -2 to +2 mEq/L
Common Imbalances
Understanding common fluid, electrolyte, and acid-base imbalances is essential for nursing practice. Each imbalance presents unique challenges requiring specific assessment and intervention strategies.
Fluid Imbalances
Fluid Volume Deficit (Hypovolemia)
Common Causes:
- • Excessive fluid losses (vomiting, diarrhea)
- • Inadequate fluid intake
- • Diuretic therapy
- • Burns, hemorrhage
- • Third-spacing (ascites, edema)
Clinical Manifestations:
- • Thirst, dry mouth
- • Decreased urine output
- • Hypotension, tachycardia
- • Poor skin elasticity
- • Confusion, weakness
Fluid Volume Excess (Hypervolemia)
Common Causes:
- • Heart failure
- • Renal failure
- • Liver disease
- • Excessive sodium intake
- • Hormonal imbalances
Clinical Manifestations:
- • Edema, weight gain
- • Jugular vein distension
- • Pulmonary edema, crackles
- • Hypertension
- • Dyspnea, orthopnea
Electrolyte Imbalances
| Imbalance | Level | Key Symptoms | ECG Changes | Priority Intervention |
|---|---|---|---|---|
| Hyponatremia | < 136 mEq/L | Confusion, seizures, muscle cramps | Usually none | Fluid restriction, hypertonic saline |
| Hypernatremia | > 145 mEq/L | Thirst, altered LOC, hyperreflexia | Usually none | Hypotonic fluids, treat underlying cause |
| Hypokalemia | < 3.5 mEq/L | Muscle weakness, paralysis, constipation | Flattened T waves, U waves | Potassium replacement, monitor cardiac |
| Hyperkalemia | > 5.0 mEq/L | Muscle weakness, paresthesias | Peaked T waves, wide QRS | Calcium gluconate, insulin/glucose |
| Hypocalcemia | < 8.5 mg/dL | Tetany, Chvostek’s sign, seizures | Prolonged QT interval | Calcium replacement, vitamin D |
| Hypermagnesemia | > 2.1 mg/dL | Decreased DTRs, respiratory depression | Prolonged PR, widened QRS | Calcium gluconate, dialysis |
Acid-Base Imbalances
Metabolic Acidosis
pH < 7.35, HCO₃⁻ < 22 mEq/L
Causes:
- • Diabetic ketoacidosis
- • Renal failure
- • Diarrhea, lactic acidosis
Compensation:
Hyperventilation (Kussmaul breathing)
Respiratory Acidosis
pH < 7.35, PCO₂ > 45 mmHg
Causes:
- • Hypoventilation
- • COPD, pneumonia
- • CNS depression
Compensation:
Renal retention of HCO₃⁻
Mnemonic: ABG Interpretation
“ROME” (Respiratory Opposite, Metabolic Equal)
- • Respiratory disorders: pH and CO₂ move in opposite directions
- • Metabolic disorders: pH and HCO₃⁻ move in the same direction
Nursing Interventions
Nursing interventions for fluid, electrolyte, and acid-base imbalances focus on restoration of balance, prevention of complications, and patient education. Interventions must be individualized based on the specific imbalance and underlying cause.
Fluid Balance Interventions
Hypovolemia Management
Immediate Actions:
- • Assess vital signs and hemodynamic status
- • Establish IV access with large-bore catheter
- • Administer prescribed fluids (crystalloids/colloids)
- • Monitor urine output (goal: >0.5 mL/kg/hr)
- • Position patient supine with legs elevated
Ongoing Monitoring:
- • Daily weights (same time, same scale)
- • Intake and output balance
- • Skin turgor and mucous membranes
- • Laboratory values (electrolytes, BUN, creatinine)
Hypervolemia Management
Immediate Actions:
- • Elevate head of bed 30-45 degrees
- • Administer prescribed diuretics
- • Restrict sodium and fluid intake as ordered
- • Monitor respiratory status closely
- • Assess for signs of pulmonary edema
Ongoing Monitoring:
- • Daily weights (expect 1-2 kg loss/day)
- • Edema assessment and documentation
- • Jugular vein distension
- • Lung sounds and oxygen saturation
IV Fluid Selection Guidelines
Isotonic Solutions
Same osmolality as plasma
- • 0.9% NaCl (Normal Saline)
- • Lactated Ringer’s
- • 5% Dextrose in Water
Use: Volume replacement, maintenance
Hypotonic Solutions
Lower osmolality than plasma
- • 0.45% NaCl (Half Normal)
- • 0.33% NaCl
- • 5% Dextrose in 0.45% NaCl
Use: Cellular hydration, hypernatremia
Hypertonic Solutions
Higher osmolality than plasma
- • 3% NaCl
- • 5% NaCl
- • 10% Dextrose in Water
Use: Severe hyponatremia, cerebral edema
Electrolyte Management
Potassium Replacement Protocol
IV Potassium Guidelines:
- • Never give IV potassium as bolus
- • Maximum concentration: 40 mEq/L peripheral
- • Maximum rate: 10 mEq/hr peripheral
- • Central line allows higher concentrations
- • Monitor cardiac rhythm continuously
Safety Considerations:
- • Check renal function before administration
- • Verify patency of IV access
- • Use infusion pump for controlled delivery
- • Monitor for phlebitis and infiltration
- • Document serial potassium levels
Mnemonic: Hyperkalemia Treatment
“C BIG K”
- • Calium gluconate (cardioprotective)
- • Bicarbonate (alkalinize)
- • Insulin + glucose (shift K+ into cells)
- • Gluctocorticoids (promote K+ excretion)
- • Kayexalate (bind K+ in GI tract)
Acid-Base Interventions
Acidosis Management
Metabolic Acidosis:
- • Treat underlying cause (DKA, renal failure)
- • Administer bicarbonate if pH < 7.1
- • Monitor for overcorrection
- • Provide respiratory support if needed
Respiratory Acidosis:
- • Improve ventilation (positioning, O₂)
- • Bronchodilators for airway obstruction
- • Mechanical ventilation if severe
- • Treat underlying respiratory condition
Alkalosis Management
Metabolic Alkalosis:
- • Correct volume depletion
- • Replace chloride deficits
- • Treat underlying cause (vomiting, diuretics)
- • Monitor potassium levels
Respiratory Alkalosis:
- • Treat anxiety or pain causing hyperventilation
- • Breathing exercises or rebreathing
- • Adjust mechanical ventilation settings
- • Address underlying hypoxia
Case Studies
Case studies provide practical application of theoretical knowledge and help develop critical thinking skills for managing complex fluid, electrolyte, and acid-base disorders.
Case Study 1: Diabetic Ketoacidosis
Patient Presentation:
22-year-old male with Type 1 diabetes presents with 3 days of nausea, vomiting, and increased urination. He appears dehydrated and has rapid, deep breathing.
Laboratory Results:
- • pH: 7.28 (acidotic)
- • PCO₂: 28 mmHg (compensated)
- • HCO₃⁻: 14 mEq/L (low)
- • Glucose: 480 mg/dL
- • Ketones: positive
- • Na⁺: 128 mEq/L, K⁺: 5.2 mEq/L
Analysis:
Metabolic acidosis with respiratory compensation. The patient has DKA with dehydration and electrolyte imbalances.
Nursing Priorities:
- • Fluid resuscitation with 0.9% NaCl
- • Insulin therapy to reverse ketosis
- • Potassium replacement (will drop with insulin)
- • Monitor for cerebral edema
- • Frequent blood glucose and ABG monitoring
Case Study 2: Heart Failure with Fluid Overload
Patient Presentation:
68-year-old female with CHF presents with increased dyspnea, orthopnea, and 5-pound weight gain over 2 days. She has bilateral lower extremity edema.
Assessment Findings:
- • Crackles bilateral lung bases
- • JVD at 45 degrees
- • 3+ pitting edema bilateral ankles
- • BP: 160/95 mmHg
- • Na⁺: 132 mEq/L, BUN: 45 mg/dL
Analysis:
Fluid volume excess secondary to heart failure exacerbation. The patient shows signs of pulmonary and peripheral edema.
Nursing Priorities:
- • HOB elevation, oxygen therapy
- • Diuretic administration (furosemide)
- • Strict I&O monitoring
- • Daily weights (same time/scale)
- • Sodium/fluid restriction
- • Monitor for electrolyte depletion
Case Study 3: Chronic Kidney Disease
Patient Presentation:
55-year-old male with CKD stage 4 presents with weakness, nausea, and metallic taste. He has been missing dialysis sessions.
Laboratory Results:
- • K⁺: 6.8 mEq/L (critically high)
- • BUN: 85 mg/dL, Creatinine: 4.2 mg/dL
- • pH: 7.32, HCO₃⁻: 16 mEq/L
- • Ca²⁺: 7.8 mg/dL, PO₄³⁻: 6.5 mg/dL
- • ECG: Peaked T waves
Analysis:
Multiple electrolyte imbalances and metabolic acidosis due to renal failure. Hyperkalemia is life-threatening.
Nursing Priorities:
- • Cardiac monitoring (hyperkalemia)
- • Calcium gluconate for cardioprotection
- • Insulin/glucose to shift K⁺ intracellularly
- • Prepare for emergent dialysis
- • Monitor for cardiac arrhythmias
- • Educate on dietary restrictions
Clinical Decision-Making Framework
When managing complex fluid, electrolyte, and acid-base disorders, follow this systematic approach:
1. Assess
Gather comprehensive data including vital signs, symptoms, and laboratory values
2. Analyze
Identify primary disorder and compensatory mechanisms
3. Prioritize
Address life-threatening imbalances first (ABCs)
4. Evaluate
Monitor response to interventions and adjust plan
Implementation in Nursing Practice
Successful implementation of fluid, electrolyte, and acid-base management requires integration of theoretical knowledge with practical skills, critical thinking, and evidence-based practice guidelines.
Clinical Practice Guidelines
Assessment Standards
Frequency of Assessment:
- • Critical patients: Every 1-2 hours
- • Acute care: Every 4-8 hours
- • Stable patients: Every 12 hours
- • Post-intervention: Within 30 minutes
Documentation Requirements:
- • Accurate intake and output
- • Daily weights (same conditions)
- • Skin turgor and edema assessment
- • Neurological status changes
Safety Protocols
High-Risk Medications:
- • Potassium: Never push IV, always use pump
- • Hypertonic saline: Central line preferred
- • Bicarbonate: Monitor for overcorrection
- • Diuretics: Monitor electrolytes closely
Emergency Protocols:
- • Hyperkalemia: Have calcium gluconate ready
- • Severe hyponatremia: Seizure precautions
- • Respiratory acidosis: Airway management
- • Fluid overload: Pulmonary edema protocol
Patient Education and Discharge Planning
Education Priorities
Dietary Modifications
- • Sodium restriction guidelines
- • Potassium-rich foods identification
- • Fluid restriction techniques
- • Reading food labels
Medication Management
- • Proper timing and dosing
- • Side effects recognition
- • Drug-food interactions
- • When to contact healthcare provider
Self-Monitoring
- • Daily weight procedures
- • Symptom recognition
- • Fluid intake tracking
- • Emergency action plans
Quality Improvement and Evidence-Based Practice
Best Practice Integration
Research-Based Interventions
- • Use of early warning systems for electrolyte monitoring
- • Implementation of fluid balance protocols
- • Standardized assessment tools for dehydration
- • Evidence-based guidelines for IV fluid selection
Quality Metrics
- • Reduction in preventable electrolyte imbalances
- • Decreased hospital readmissions for fluid overload
- • Improved patient satisfaction scores
- • Enhanced staff competency in fluid management
Interdisciplinary Collaboration
Effective management of fluid, electrolyte, and acid-base disorders requires seamless collaboration among healthcare team members:
Team Communication
- • Physicians: Collaborate on treatment plans and medication adjustments
- • Pharmacists: Consult on drug interactions and dosing protocols
- • Dietitians: Coordinate dietary modifications and education
- • Laboratory: Ensure timely and accurate test results
Care Coordination
- • Handoff Communication: SBAR format for shift changes
- • Discharge Planning: Coordinate home care and follow-up
- • Emergency Response: Rapid response team activation
- • Continuous Monitoring: 24/7 surveillance protocols
Implementation Success Factors
“SAFE CARE”
- • Systematic assessment protocols
- • Accurate documentation practices
- • Frequent monitoring schedules
- • Evidence-based interventions
- • Continuous education and training
- • Adherence to safety protocols
- • Rapid response to changes
- • Effective team communication
Conclusion
Mastery of fluid, electrolyte, and acid-base balance is fundamental to nursing practice across all healthcare settings. This comprehensive review has provided evidence-based knowledge and practical applications necessary for safe, effective patient care.
Key Takeaways
- • Understanding physiological regulation mechanisms is essential for recognizing and managing imbalances
- • Systematic assessment and early intervention prevent complications and improve patient outcomes
- • Integration of theoretical knowledge with clinical skills enhances critical thinking abilities
- • Interdisciplinary collaboration and evidence-based practice optimize patient care delivery
- • Continuous learning and competency development ensure safe, quality nursing practice
Future Considerations
As healthcare continues to evolve, nurses must stay current with emerging research and technological advances in fluid, electrolyte, and acid-base management:
- • Point-of-care testing for rapid diagnosis
- • Advanced monitoring technologies
- • Precision medicine approaches
- • Artificial intelligence-assisted decision support
- • Enhanced patient education platforms
Committed to excellence in nursing education and evidence-based practice
