Prevention of Needle-Stick Injuries
A Comprehensive Nursing Guide for Healthcare Safety
Introduction & Critical Statistics
Needle-stick injuries represent one of the most significant occupational hazards in healthcare settings. These preventable incidents affect thousands of healthcare workers annually and pose serious risks of bloodborne pathogen transmission. Understanding comprehensive prevention strategies is crucial for every nursing professional.
600,000+
Annual needle-stick injuries in US healthcare workers
60+
Pathogens that can be transmitted through needle-stick injuries
80%
Reduction possible with proper prevention strategies
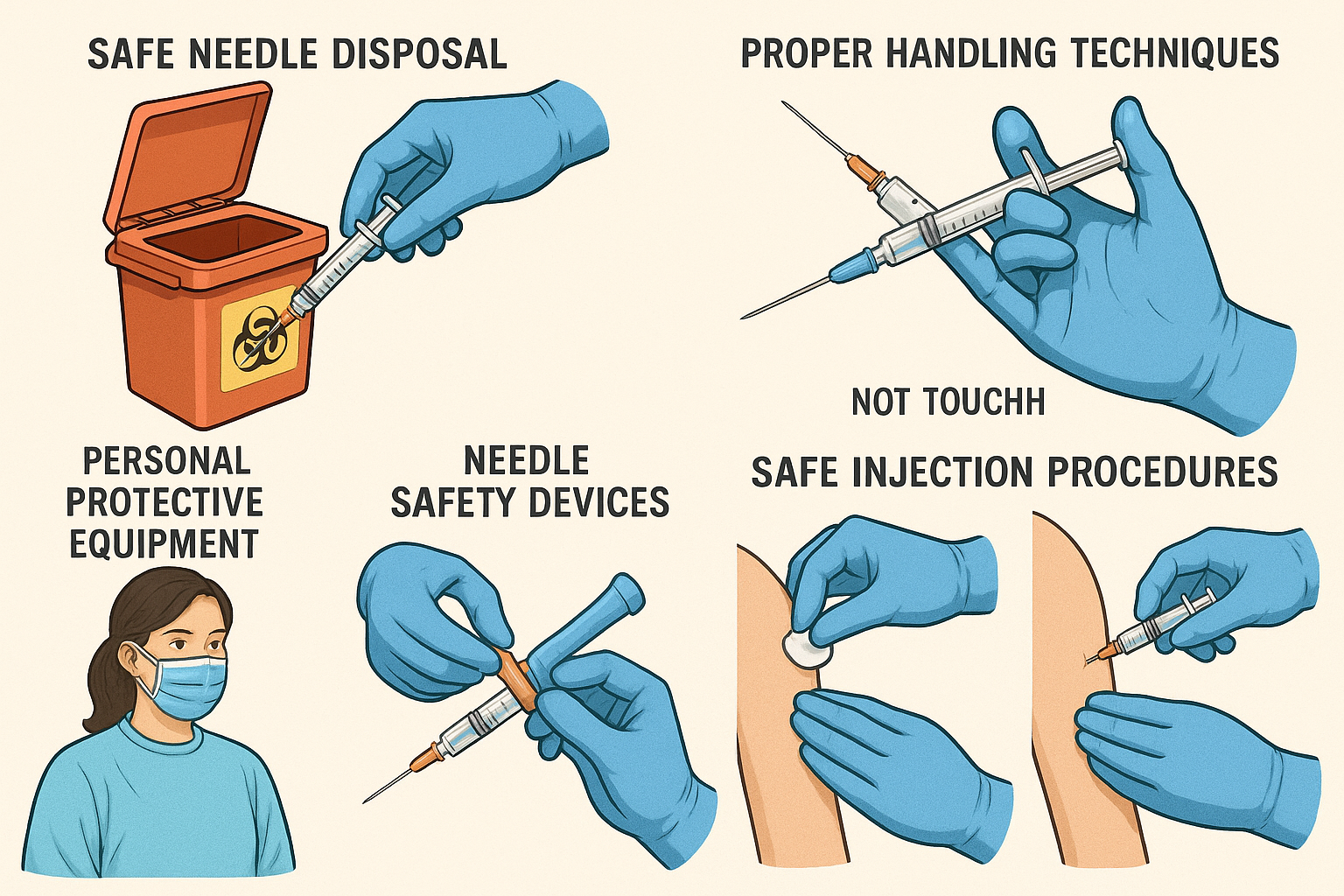
Comprehensive needle-stick injury prevention techniques and safety protocols
The Human Cost
- 1,000 healthcare workers infected with HIV annually due to occupational exposure
- 16,000 hepatitis C infections among healthcare workers yearly
- Average cost per needle-stick injury: $3,000-$5,000
- Psychological impact: anxiety, depression, and PTSD in affected workers
Understanding Needle-Stick Injuries
Definition & Types
A needle-stick injury occurs when a needle or sharp medical instrument penetrates the skin of a healthcare worker, potentially exposing them to bloodborne pathogens.
Types of Sharp Injuries:
- Hypodermic needles: Most common type
- Blood collection needles: High contamination risk
- Scalpels: Deep penetration potential
- Laboratory glass: Multiple exposure sites
- IV catheter needles: Common in bedside care
Pathogen Transmission Risk
High Risk Pathogens
- Hepatitis B Virus (HBV) – 30% transmission risk
- Hepatitis C Virus (HCV) – 1.8% transmission risk
- Human Immunodeficiency Virus (HIV) – 0.3% transmission risk
Other Concerning Pathogens
- Staphylococcus aureus (including MRSA)
- Streptococcus pyogenes
- Cytomegalovirus (CMV)
- Epstein-Barr Virus (EBV)
Factors Affecting Transmission Risk
| Factor | High Risk | Low Risk | Impact on Transmission |
|---|---|---|---|
| Needle Type | Large bore, hollow needles | Small gauge, solid needles | Larger volume of blood transfer |
| Depth of Injury | Deep penetration | Superficial scratch | Deeper wounds allow more pathogen entry |
| Source Patient Status | High viral load, acute infection | Low/undetectable viral load | Higher viral loads increase transmission probability |
| Time to Treatment | Delayed response (>72 hours) | Immediate treatment (<4 hours) | Early intervention reduces infection risk |
| Blood Visibility | Visible blood on device | No visible blood | More blood equals higher pathogen load |
Risk Factors & High-Risk Situations
Personnel Factors
- Inexperienced staff
- Fatigue and overwork
- Stress and time pressure
- Inadequate training
- Complacency
Environmental Factors
- Poor lighting conditions
- Crowded work spaces
- Inadequate sharps containers
- Emergency situations
- Understaffed units
Procedural Factors
- Recapping needles
- Passing sharp instruments
- Improper disposal
- Rushing through procedures
- Working on unstable patients
High-Risk Situations in Clinical Practice
Medication Administration
Drawing medications from vials, administering injections, and IV therapy procedures account for 35% of needle-stick injuries.
Blood Collection & Laboratory Procedures
Venipuncture, arterial blood draws, and specimen handling represent 28% of occupational needle-stick injuries.
Surgical & Invasive Procedures
Operating rooms and procedural areas pose significant risks, especially during instrument passing and suturing.
Waste Management & Disposal
Improper disposal practices and overfilled sharps containers contribute to 15% of needle-stick injuries.
Personal Risk Assessment Checklist
High-Risk Indicators
- Working >12 hour shifts regularly
- Less than 6 months experience
- Working in ICU, OR, or ED
- Performing frequent invasive procedures
- Limited safety device availability
Protective Factors
- Regular safety training participation
- Use of safety-engineered devices
- Adequate staffing levels
- Strong safety culture in workplace
- Access to immediate post-exposure care
Comprehensive Prevention Strategies
The Three-Tier Prevention Approach
Effective needle-stick injury prevention requires a multi-layered approach combining engineering controls, administrative measures, and personal protective practices.
Primary Prevention: Engineering Controls
Safety-Engineered Devices
-
Retractable needles: Needle retracts into syringe after use
-
Needle guards: Protective sheath covers needle automatically
-
Blunt-tip needles: For drawing medications from vials
-
Self-sheathing IV catheters: Automatic needle protection
Sharps Disposal Systems
-
Puncture-resistant containers: FDA-approved sharps containers
-
Point-of-use placement: Containers within arm’s reach
-
Clear visibility: Easy identification of fill level
-
Secure closure: Tamper-resistant locking mechanism
Secondary Prevention: Administrative Controls
Policy Development & Implementation
- Comprehensive sharps safety policy
- Incident reporting procedures
- Post-exposure protocols
- Regular policy review and updates
- Staff training requirements
- Equipment evaluation procedures
- Quality assurance measures
- Compliance monitoring systems
Training Programs
- • Annual safety education
- • Device-specific training
- • Simulation exercises
- • Competency validation
Surveillance Systems
- • Injury tracking database
- • Trend analysis reporting
- • Risk factor identification
- • Performance metrics
Culture Development
- • Leadership commitment
- • Open reporting environment
- • Peer accountability
- • Continuous improvement
Tertiary Prevention: Personal Protective Practices
Safe Handling Techniques
Personal Protective Equipment
Gloves
Nitrile or latex examination gloves for all procedures involving potential blood contact
Eye Protection
Safety glasses or face shields when splash risk is present
Protective Clothing
Fluid-resistant gowns for procedures with high contamination risk
Respiratory Protection
Surgical masks or N95 respirators based on transmission risk assessment
Safety Devices & Technology Innovations
Retractable Syringes
Needle automatically retracts into barrel after injection, eliminating exposure risk.
Safety Needles
Integrated safety mechanisms including sliding shields and hinged caps.
Blunt-Tip Devices
Needles with blunted tips for medication preparation and IV access.
Safety Device Effectiveness Comparison
| Device Type | Injury Reduction | Cost Factor | User Acceptance | Best Applications |
|---|---|---|---|---|
| Retractable Syringes | 95% | High (3-5x cost) | Excellent | Immunizations, insulin administration |
| Safety IV Catheters | 85% | Moderate (2x cost) | High | IV insertion, blood draws |
| Hinged Safety Needles | 78% | Low (1.5x cost) | Moderate | General injections, lab draws |
| Blunt-Tip Needles | 92% | Very Low (1.2x cost) | Excellent | Medication preparation, IV access |
| Sliding Shield Devices | 82% | Moderate (2.5x cost) | Variable | Blood collection, arterial puncture |
Emerging Technologies in Needle Safety
Smart Safety Systems
- RFID-enabled sharps containers: Real-time fill-level monitoring
- Mobile safety apps: Incident reporting and training modules
- Computer vision: AI-powered safety compliance monitoring
- Predictive analytics: Risk assessment algorithms
Advanced Materials
- Biodegradable needles: Dissolving needle technology
- Self-healing materials: Puncture-resistant containers
- Microneedle patches: Pain-free medication delivery
- Temperature-sensitive polymers: Smart activation systems
Standard Protocols & Procedures
Safe Injection Protocol: Step-by-Step Guide
Pre-Injection Phase
Hand Hygiene & PPE
Perform hand hygiene and don appropriate personal protective equipment including gloves
Equipment Selection
Choose appropriate safety-engineered device and verify sharps container proximity
Medication Preparation
Use blunt-tip needles for drawing medications, maintain sterile technique throughout
Patient Positioning
Ensure stable patient position and optimal lighting conditions for the procedure
Injection & Post-Procedure
Safe Injection Technique
Maintain needle visibility, avoid sudden movements, communicate with patient throughout
Immediate Safety Activation
Activate safety mechanism immediately after withdrawal, before moving needle
Proper Disposal
Place entire device directly into sharps container without disassembly
Documentation
Complete procedure documentation and remove PPE following proper sequence
Sharps Container Management Protocol
Placement Guidelines
- • Within arm’s reach of work area
- • Eye level or below for visibility
- • Stable, flat surface mounting
- • Away from patient traffic areas
- • Multiple containers for high-volume areas
Fill Level Monitoring
- • Check fill level before each use
- • Replace when 3/4 full (never overfill)
- • Visual inspection for damage
- • Clear fill-line markings
- • Documentation of replacement dates
Secure Disposal
- • Permanent closure mechanism
- • Tamper-evident sealing
- • Authorized personnel handling only
- • Proper labeling and tracking
- • Licensed disposal service
Never Do These Actions – Critical Safety Violations
Needle Handling
- Never recap needles using both hands
- Never bend or break needles manually
- Never remove needles from disposable syringes
- Never pass sharp instruments hand-to-hand
Disposal Practices
- Never overfill sharps containers
- Never reach into sharps containers
- Never place sharps in regular waste
- Never transport unprotected sharps
Post-Exposure Management Protocol
Time-Critical Response: First 60 Minutes
Immediate action within the first hour post-exposure is crucial for optimal outcomes. Every minute counts in preventing potential infections from needle-stick injuries.
Immediate Response Actions (0-15 minutes)
Step 1: Wound Care (Immediate)
- • Allow wound to bleed freely for 30 seconds
- • Wash thoroughly with soap and water for 2 minutes
- • Do NOT squeeze or manipulate the wound
- • Apply antiseptic if available (70% alcohol or iodine)
Step 2: Immediate Notification (Within 5 minutes)
- • Notify supervisor or charge nurse immediately
- • Contact occupational health or emergency department
- • Begin incident documentation process
- • Preserve the sharp device if safely possible
Step 3: Source Assessment (Within 15 minutes)
- • Identify source patient if known
- • Review patient’s infection status and risk factors
- • Collect source patient blood samples (if consented)
- • Document circumstances of exposure
Risk Assessment & Laboratory Testing Protocol
Exposed Healthcare Worker Testing
Baseline Testing (Day 0)
- • HIV antibody test
- • Hepatitis B surface antibody
- • Hepatitis C antibody
- • Complete blood count
- • Liver function tests
Follow-up Testing Schedule
- • 6 weeks: HIV, HCV antibodies
- • 12 weeks: HIV, HCV antibodies, LFTs
- • 6 months: HIV, HCV antibodies (final)
- • HBV testing if not immune
Source Patient Evaluation
Required Testing (if consent obtained)
- • HIV antibody and viral load
- • Hepatitis B surface antigen
- • Hepatitis C antibody and PCR
- • Review medical history for risk factors
Unknown Source Protocol
- • Assess exposure circumstances
- • Consider local epidemiology data
- • Evaluate need for prophylaxis
- • Enhanced surveillance protocols
Post-Exposure Prophylaxis (PEP) Decision Matrix
| Pathogen | Source Status | Recommended PEP | Duration | Efficacy |
|---|---|---|---|---|
| HIV | High viral load | 3-drug combination therapy | 28 days | 80% effective |
| HIV | Undetectable viral load | Consider case-by-case | 28 days | Not usually required |
| Hepatitis B | HBsAg positive | HBIG + vaccine series | Immediate + 6 months | 95% effective |
| Hepatitis C | HCV positive | No prophylaxis available | Monitoring only | Early treatment if infection occurs |
Psychological Support & Counseling
Immediate Support Services
- 24/7 counseling hotline access
- Peer support group referrals
- Scheduled follow-up appointments
- Educational materials and resources
Long-term Mental Health
- Anxiety and depression screening
- PTSD assessment and treatment
- Return-to-work counseling
- Family counseling services
Memory Aids & Mnemonics for Needle Safety
S.H.A.R.P Safety Protocol
Safety First
Always choose safety-engineered devices when available
Handle with Care
Maintain needle visibility and avoid sudden movements
Activate Immediately
Engage safety mechanism right after use
Refuse to Recap
Never recap needles using two-handed technique
Place in Container
Dispose directly into sharps container immediately
N.E.E.D.L.E Post-Exposure Response
Notify Immediately
Report to supervisor within 5 minutes
Encourage Bleeding
Allow wound to bleed freely for 30 seconds
Evaluate Source
Assess source patient infection status
Document Everything
Complete incident report thoroughly
Laboratory Testing
Obtain baseline and follow-up labs
Engage Support
Access counseling and peer support
“Never, Never, Never”
- • Never recap with both hands
- • Never bend needles manually
- • Never reach into sharps containers
“Golden Hour Rule”
The first 60 minutes after a needle-stick injury are critical. Quick action can reduce transmission risk by up to 80%.
“3/4 Full Rule”
Always replace sharps containers when they reach 3/4 capacity. Overfilling increases injury risk exponentially.
Visual Memory Aid: Safety Colors
RED
Danger/Stop
YELLOW
Caution/Think
GREEN
Safe/Go
BLUE
Information/Help
Education & Training Programs
Evidence-Based Training Approach
Effective needle-stick injury prevention training combines theoretical knowledge with hands-on practice, utilizing adult learning principles and behavior change theories for maximum retention and application.
Comprehensive Training Program Components
Didactic Learning
- • Epidemiology and risk factors
- • Pathogen transmission routes
- • Safety device mechanisms
- • Regulatory requirements
- • Post-exposure protocols
Skills Training
- • Safe injection techniques
- • Device activation practice
- • Proper disposal methods
- • Emergency response drills
- • Wound care procedures
Simulation
- • High-fidelity scenarios
- • Stress-testing protocols
- • Decision-making practice
- • Team-based exercises
- • Error recognition training
Assessment
- • Competency validation
- • Knowledge testing
- • Skill demonstration
- • Performance metrics
- • Continuing education
Core Learning Objectives
Knowledge Objectives
- • Identify risk factors for needle-stick injuries in clinical practice
- • Describe transmission risks for bloodborne pathogens
- • Explain mechanisms of safety-engineered devices
- • Outline post-exposure management protocols
- • Recognize legal and regulatory requirements
Skill Objectives
- • Demonstrate proper use of safety-engineered devices
- • Perform safe injection and blood collection techniques
- • Execute appropriate sharps disposal procedures
- • Apply immediate post-exposure wound care
- • Complete accurate incident documentation
Attitude Objectives
- • Value the importance of universal safety precautions
- • Commit to consistent use of safety devices
- • Promote a culture of safety among peers
- • Accept responsibility for personal and patient safety
- • Embrace continuous learning and improvement
Traditional Methods
Classroom Instruction
Interactive lectures, group discussions, and case study analysis
Hands-on Workshops
Practical device training and skill demonstration sessions
Peer Learning
Experienced staff mentoring and buddy system implementation
Innovative Approaches
E-Learning Platforms
Interactive modules with immediate feedback and progress tracking
Virtual Reality
Immersive training environments for high-risk scenarios
Gamification
Learning games, competitions, and achievement systems
Legal & Regulatory Framework
Legal Imperatives
Healthcare facilities are legally mandated to provide safe working environments and implement comprehensive needle-stick injury prevention programs. Non-compliance can result in significant legal and financial consequences.
Major Regulatory Requirements
OSHA Bloodborne Pathogens Standard (29 CFR 1910.1030)
Core Requirements
- • Written exposure control plan
- • Annual review and update
- • Employee training programs
- • Hepatitis B vaccination
- • Post-exposure evaluation
Safety Requirements
- • Engineering controls implementation
- • Work practice controls
- • Personal protective equipment
- • Sharps injury log maintenance
- • Safety device evaluation
Needlestick Safety and Prevention Act (2000)
Key Provisions
- • Safety-engineered device use
- • Sharps injury log requirements
- • Employee input in device selection
- • Annual plan reviews
- • Injury data analysis
Documentation Requirements
- • Detailed injury circumstances
- • Device type and brand
- • Department and work area
- • Injury severity assessment
- • Prevention recommendations
Joint Commission Standards
Safety Goals
- • Improve staff safety culture
- • Reduce occupational injuries
- • Enhance reporting systems
- • Implement evidence-based practices
- • Monitor safety performance
Compliance Elements
- • Leadership commitment
- • Policy development
- • Staff education
- • Performance measurement
- • Continuous improvement
Regulatory Compliance Checklist
Documentation Requirements
Program Implementation
Consequences of Non-Compliance
Financial Penalties
- • OSHA fines up to $136,532 per violation
- • Increased workers’ compensation costs
- • Legal settlements and judgments
- • Loss of accreditation
Operational Impact
- • Work stoppage orders
- • Increased regulatory oversight
- • Staff turnover and recruitment issues
- • Negative public relations
Human Impact
- • Employee injuries and infections
- • Reduced staff morale
- • Loss of public trust
- • Personal liability for administrators
Case Studies & Real-World Examples
Case Study 1: Emergency Department Needle-Stick Incident
Incident Description
Setting: Urban hospital emergency department, night shift
Staff: 2nd-year nursing student during clinical rotation
Patient: 45-year-old homeless individual with unknown HIV status
Procedure: IV insertion for trauma workup
Incident: Student attempted to recap needle using two-handed method, sustained deep puncture to thumb
Contributing Factors
- Inadequate supervision of student
- Non-safety IV catheter used
- High-stress emergency environment
- Poor lighting in patient bay
- Sharps container not within reach
Lessons Learned & Prevention Strategies
Immediate Changes
- • Mandatory safety IV catheters
- • Enhanced student supervision
- • Additional sharps containers
Training Improvements
- • Hands-on safety device training
- • Stress management techniques
- • Never-recap policy reinforcement
System Changes
- • LED task lighting installation
- • Student competency validation
- • Incident reporting improvements
Case Study 2: Successful Prevention Program Implementation
Hospital Profile
Setting: 500-bed academic medical center
Challenge: 150+ needle-stick injuries annually
Timeline: 3-year implementation period
Investment: $2.1 million in safety devices and training
Results: 87% reduction in injuries
Key Success Factors
- Strong executive leadership support
- Comprehensive staff engagement
- Phased safety device implementation
- Intensive training programs
- Robust data tracking systems
Implementation Timeline & Results
| Phase | Duration | Key Activities | Injury Reduction |
|---|---|---|---|
| Phase 1 | 6 months | Leadership buy-in, baseline assessment | 0% |
| Phase 2 | 12 months | Device pilot programs, staff training | 25% |
| Phase 3 | 18 months | Full implementation, culture change | 87% |
Case Study 3: Innovative Technology Solution
Technology Innovation
Hospital: 200-bed community hospital
Innovation: RFID-enabled sharps containers
Features: Real-time fill monitoring, automated alerts
Integration: Hospital information system connectivity
Cost: $50,000 initial investment
Measured Outcomes
- 65% reduction in overfilled containers
- 40% improvement in replacement timing
- 23% decrease in disposal-related injuries
- ROI achieved within 18 months
- Improved staff satisfaction scores
Scalability and Adoption
The success of this pilot program led to health system-wide adoption across 12 facilities, demonstrating the potential for technology-driven safety improvements in healthcare settings.
Implementation Keys
- • Staff buy-in and engagement
- • Comprehensive training program
- • Phased rollout approach
- • Continuous monitoring and adjustment
Future Enhancements
- • AI-powered risk prediction
- • Mobile app integration
- • Predictive analytics dashboard
- • Automated compliance reporting
