Step-by-Step Process of Embryological Development and Placental Formation
A comprehensive guide for nursing students
Table of Contents
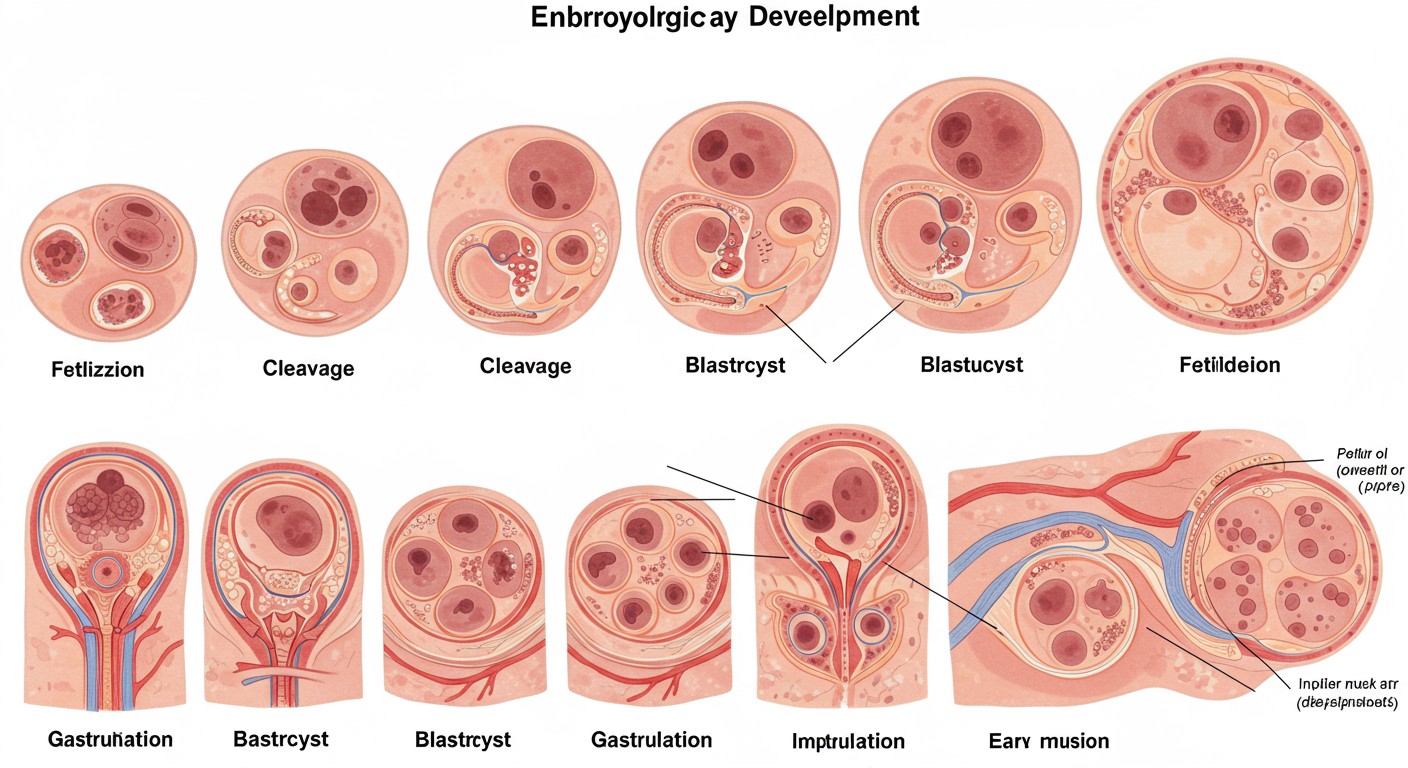
Comprehensive illustration of embryological development and placental formation stages
1. Introduction to Embryological Development
Embryological development is the complex process through which a single-celled zygote transforms into a multi-cellular organism with differentiated tissues and organ systems. This fascinating journey spans from conception to birth, with the most critical developmental events occurring during the first eight weeks—the embryonic period.
For nursing students, understanding embryological development is crucial as it provides the foundation for comprehending normal anatomy, congenital disorders, and developmental abnormalities. The knowledge of normal development helps in identifying and addressing pathological conditions that may arise due to developmental disruptions.
Key Point
Human embryological development is traditionally divided into three trimesters, but the most critical development occurs during the first trimester, particularly the first 8 weeks (embryonic period). After 8 weeks, the developing human is referred to as a fetus.
This comprehensive guide will walk you through the step-by-step process of embryological development and placental formation, providing clear explanations of complex concepts and practical mnemonics to aid your learning journey.
2. Pre-implantation Development
The journey of embryological development begins with fertilization and proceeds through several critical stages before the embryo implants into the uterine wall. These pre-implantation stages are fundamental to successful development.
2.1 Fertilization
Fertilization is the process where a haploid sperm (containing 23 chromosomes) fuses with a haploid oocyte (containing 23 chromosomes) to form a diploid zygote (46 chromosomes). This remarkable event typically occurs in the ampullary region of the fallopian tube.
Steps of Fertilization:
- Capacitation: Sperm undergo biochemical changes in the female reproductive tract, enabling them to fertilize the oocyte.
- Acrosome Reaction: The acrosomal cap of the sperm releases enzymes to digest the corona radiata and zona pellucida.
- Sperm-Oocyte Fusion: A single sperm fuses with the oocyte membrane.
- Cortical Reaction: Prevents polyspermy by hardening the zona pellucida.
- Completion of Meiosis II: The oocyte completes its second meiotic division.
- Pronuclear Formation: Male and female pronuclei form and migrate toward each other.
- Syngamy: The genetic material of both gametes combines, forming a diploid zygote.
Barriers Overcome by Sperm:
- Corona Radiata: Layer of follicular cells surrounding the oocyte
- Zona Pellucida: Glycoprotein layer below the corona radiata
- Vitelline Membrane: The plasma membrane of the oocyte
Significance for Nursing:
Understanding fertilization helps explain ectopic pregnancies, infertility issues, and the effects of contraceptive methods that prevent fertilization.
Clinical Connection
Abnormalities in fertilization can lead to various conditions such as triploidy (69 chromosomes) which occurs when two sperm fertilize one egg or when a diploid sperm fertilizes a haploid egg. Most triploid embryos result in early spontaneous abortion.
2.2 Cleavage
Cleavage refers to the rapid mitotic divisions of the zygote without an increase in cytoplasmic volume. These divisions produce progressively smaller cells called blastomeres.
| Stage | Timing Post-Fertilization | Description |
|---|---|---|
| Zygote | Day 0-1 | Single-celled diploid cell formed after fertilization |
| 2-Cell Stage | Day 1-2 | First cleavage division; cells are totipotent |
| 4-Cell Stage | Day 2 | Second cleavage division; still totipotent |
| 8-Cell Stage | Day 3 | Third cleavage division; totipotency diminishes |
| 12-16 Cell Stage | Day 3-4 | Continued division; approaching morula stage |
During cleavage, the embryo remains enclosed within the zona pellucida and begins its journey from the fallopian tube toward the uterine cavity. These early divisions are critical as they establish the foundation for subsequent development.
Key Point
In the early stages of cleavage, cells are totipotent, meaning each blastomere has the potential to develop into a complete embryo if separated. This explains the formation of identical twins when separation occurs during this period.
2.3 Morula Formation
By approximately day 4 post-fertilization, the embryo has undergone several cleavage divisions, resulting in a solid ball of 16-32 cells called a morula (Latin for “mulberry”). The morula forms as the embryo continues its journey through the fallopian tube toward the uterine cavity.
Key Characteristics of the Morula:
- Solid ball of cells (16-32 cells)
- Still enclosed within zona pellucida
- Cells begin to compact and form tight junctions
- Cell differentiation begins with inner and outer cell populations
- Still contained within the zona pellucida
Developmental Significance:
The morula stage represents a critical transition point in embryological development where:
- Cells become more tightly packed through compaction
- E-cadherin and Ca²⁺-driven signal transduction drive cell boundary changes
- Initial cell fate determination begins
- First signs of polarization appear, with inner cells differing from outer cells
Compaction in the morula is a crucial process where cell boundaries begin to disappear, and cells adhere more tightly to one another. This compaction process is driven by E-cadherins and calcium-dependent cellular adhesion molecules. As the morula develops, outer cells begin to express Na⁺ transporters, preparing for the next stage of development—blastocyst formation.
2.4 Blastocyst Formation
By approximately day 5 post-fertilization, the morula transforms into a blastocyst—a hollow sphere with an inner cell mass, a fluid-filled cavity (blastocoel), and an outer layer of cells (trophoblast). This transformation marks a significant step in embryological development as distinct cell lineages become established.
Components of the Blastocyst:
- Inner Cell Mass (Embryoblast): Cluster of cells that will develop into the embryo proper
- Blastocoel: Fluid-filled cavity created by active transport of ions and water
- Trophoblast (Trophectoderm): Outer layer of cells that will contribute to placenta formation
- Zona Pellucida: Gradually thinning protective layer that will eventually rupture
Blastocyst Development Process:
- Outer morula cells develop Na⁺/K⁺-ATPase pumps
- Sodium ions are actively pumped into the interior of the morula
- Water follows sodium by osmosis, creating the blastocoel cavity
- The blastocyst expands as fluid accumulates
- The zona pellucida thins and eventually ruptures (hatching)
- The “hatched” blastocyst is now ready for implantation
Key Point
The first cell differentiation in embryological development occurs at the blastocyst stage with the formation of the inner cell mass (which becomes the embryo) and the trophoblast (which contributes to the placenta). Transcription factors Oct4, Nanog, and Sox2 maintain pluripotency in the inner cell mass, while Cdx2 and Eomes direct trophoblast development.
Clinical Connection
In vitro fertilization (IVF) procedures typically involve blastocyst culture followed by blastocyst transfer to the uterus. Understanding blastocyst development is essential for evaluating embryo quality in fertility treatments. Blastocysts are graded based on inner cell mass quality, trophectoderm quality, and expansion status.
3. Implantation
Implantation is the process by which the blastocyst embeds itself into the endometrial lining of the uterus. This critical step in embryological development typically occurs between days 6-10 post-fertilization and establishes the maternal-fetal interface necessary for further development.
Stages of Implantation:
- Hatching: The blastocyst escapes from the zona pellucida
- Apposition: Initial contact between the blastocyst and endometrium
- Adhesion: Stable attachment of the blastocyst to the endometrium
- Invasion: Trophoblast cells penetrate into the endometrium
- Decidualization: Transformation of endometrial stromal cells
Endometrial Requirements for Implantation:
- Receptive Endometrium: Typically days 20-24 of menstrual cycle (LH+7 to LH+11)
- Secretory Phase: Characterized by high progesterone levels
- Pinopodes: Endometrial surface projections that facilitate adhesion
- Adequate Vascularization: Ensures sufficient nutrient supply
- Immunological Tolerance: Prevents rejection of the embryo
The successful implantation of the blastocyst involves precise coordination between the embryo and the mother’s endometrium. The blastocyst typically implants in the upper posterior wall of the uterus. The inner cell mass is positioned toward the endometrium, while the trophoblast initiates the invasion process.
As implantation progresses, the trophoblast differentiates into two layers:
- Cytotrophoblast: Inner layer of mononucleated cells that maintain the capacity for mitotic division
- Syncytiotrophoblast: Outer multinucleated layer formed by the fusion of cytotrophoblast cells; lacks cell boundaries and is responsible for endometrial invasion and hormone production
Key Point
The syncytiotrophoblast produces human chorionic gonadotropin (hCG), which maintains the corpus luteum and prevents menstruation. This is the hormone detected in pregnancy tests. The syncytiotrophoblast also secretes proteolytic enzymes that digest the endometrial matrix, facilitating invasion.
Clinical Connection
Implantation bleeding, experienced by about 25% of pregnant women, is mild spotting that occurs when the blastocyst implants in the endometrium. This is normal and should not be confused with menstruation. Abnormal implantation sites can lead to ectopic pregnancies, with tubal pregnancies being the most common type (about 95% of ectopic pregnancies).
By the end of implantation (approximately day 10-12), the blastocyst is completely embedded in the endometrium, and the endometrial epithelium has grown over the site of implantation. The maternal blood vessels are eroded by the invasive syncytiotrophoblast, creating lacunae that fill with maternal blood—the beginning of the uteroplacental circulation.
4. Gastrulation
Gastrulation is a pivotal moment in embryological development occurring during week 3 (days 14-21), where the bilaminar embryonic disc reorganizes to form three distinct germ layers. This process establishes the foundation for all future organ systems and body structures.
4.1 Formation of Germ Layers
Prior to gastrulation, the embryo consists of two layers:
- Epiblast: Upper layer that gives rise to all three germ layers
- Hypoblast: Lower layer that contributes to extraembryonic structures
Gastrulation begins with the formation of the primitive streak on the surface of the epiblast. This linear band of cells appears at the caudal end of the embryonic disc and extends cranially, with a depression called the primitive groove running along its length.
Key Structures in Gastrulation:
- Primitive Streak: Linear band of cells marking the embryo’s longitudinal axis
- Primitive Node: Thickened cranial end of the streak
- Primitive Groove: Depression along the primitive streak
- Primitive Pit: Depression at the primitive node
- Notochordal Process: Rod-like structure extending from the primitive node toward the cranial end
Cell Migration During Gastrulation:
- Epiblast cells migrate toward the primitive streak
- Cells undergo an epithelial-to-mesenchymal transition
- Cells invaginate through the primitive streak
- The first invaginating cells displace the hypoblast to form endoderm
- Later invaginating cells spread between epiblast and endoderm to form mesoderm
- Remaining epiblast cells become ectoderm
The result of gastrulation is the establishment of three distinct germ layers:
- Ectoderm: Outermost layer derived from epiblast cells that don’t migrate
- Mesoderm: Middle layer formed by cells that migrate through the primitive streak and spread between the other layers
- Endoderm: Innermost layer formed by the first cells migrating through the primitive streak
Mnemonic: “EME” (Outside → Inside)
Ectoderm: External layer (outermost)
Mesoderm: Middle layer
Endoderm: Enteric layer (innermost)
Key Point
The primitive streak determines the longitudinal axis and establishes bilateral symmetry in the embryo. The appearance of the primitive streak marks the start of gastrulation and allows us to identify the embryo’s cranial-caudal, left-right, and dorsal-ventral axes.
4.2 Germ Layer Derivatives
Each of the three germ layers established during gastrulation gives rise to specific tissues and organs in the developing embryo:
| Germ Layer | Major Derivatives | Examples of Structures |
|---|---|---|
| Ectoderm |
– Central nervous system – Peripheral nervous system – Epidermis and epidermal derivatives – Sensory epithelium – Neural crest derivatives |
– Brain and spinal cord – Sensory ganglia and nerves – Skin, hair, nails, sweat glands – Lens, cornea, retina – Melanocytes, facial bones, odontoblasts |
| Mesoderm |
– Skeletal system – Muscular system – Cardiovascular system – Urogenital system – Connective tissue |
– Bones (except facial), cartilage – Skeletal, cardiac, smooth muscle – Heart, blood vessels, blood cells – Kidneys, gonads, genital ducts – Dermis, connective tissue |
| Endoderm |
– Digestive tract epithelium – Respiratory tract epithelium – Endocrine glands – Urinary bladder epithelium – Tympanic cavity and auditory tube |
– Stomach, intestines, liver, pancreas – Trachea, bronchi, lungs – Thyroid, parathyroid, thymus – Bladder lining – Middle ear lining |
Mnemonic: Germ Layer Derivatives
Ectoderm: “SEEN”
Skin and sensory epithelium
Epidermal derivatives (hair, nails, sweat glands)
Entire nervous system (central and peripheral)
Neural crest derivatives
Mesoderm: “MUSCLE”
Muscles (all types)
Urogenital system
Skeletal system (except facial bones)
Cardiovascular system
Lining of body cavities (peritoneum, pleura, pericardium)
Everything else (connective tissue, blood)
Endoderm: “DIRT”
Digestive tract epithelium
Internal glands (liver, pancreas, thyroid)
Respiratory tract epithelium
Tympanic cavity and auditory tube
Clinical Connection
Disruptions during gastrulation can lead to severe congenital anomalies. For example, abnormal notochord development can result in vertebral malformations such as spina bifida. Understanding the embryological origins of tissues helps explain why certain teratogens affect specific organ systems based on the timing of exposure.
5. Neurulation
Neurulation is the process by which the neural tube—the precursor to the central nervous system—forms from the ectodermal germ layer. This critical stage in embryological development occurs during weeks 3-4 and involves a series of coordinated morphological changes in the ectoderm.
Primary Neurulation (Weeks 3-4):
- Neural Plate Formation: Thickening of the midline ectoderm induced by the underlying notochord
- Neural Fold Formation: Elevation of the lateral edges of the neural plate
- Neural Groove Deepening: Formation of a midline groove as the neural folds continue to elevate
- Neural Tube Closure: Fusion of the neural folds at the dorsal midline, creating a hollow neural tube
- Neural Crest Formation: Migration of cells from the neural fold margins
Secondary Neurulation (Week 5+):
Occurs in the caudal portion of the embryo (lower sacral and coccygeal regions)
- Condensation of mesenchymal cells forms a solid cord of cells
- Cavitation within this cord creates a lumen
- The lumen connects with the neural canal formed by primary neurulation
Neural Tube Closure Sites:
Neural tube closure begins at multiple sites and proceeds in both cranial and caudal directions:
- Site 1: Cervical region (day 22)
- Site 2: Prosencephalon/mesencephalon junction
- Site 3: Rostral end of neuraxis
- Site 4: Caudal neuropore (day 26)
As neurulation progresses, the neural tube differentiates into the central nervous system, with the cranial portion forming the brain and the caudal portion forming the spinal cord. Simultaneously, neural crest cells—a special population of cells originating from the neural folds—migrate extensively throughout the embryo and differentiate into a wide variety of structures.
Key Point
Neural crest cells are often referred to as the “fourth germ layer” due to their remarkable migratory capabilities and diverse developmental fates. These cells contribute to the peripheral nervous system (sensory, sympathetic, and parasympathetic ganglia), adrenal medulla, melanocytes, and craniofacial structures.
Mnemonic: Neural Crest Derivatives “CA MOTEL ASS”
Craniofacial structures (facial bones, cartilage)
Arachnoid and pia mater
Melanocytes
Odontoblasts (dentin-forming cells)
Thymus (stromal cells)
Endocrine cells (adrenal medulla, calcitonin-producing cells)
Lingual tissues (some tongue components)
Autonomic ganglia
Sensory ganglia
Schwann cells
Clinical Connection
Neural tube defects (NTDs) result from failure of neural tube closure and are among the most common birth defects (1 in 1,000 live births). Major types include:
- Anencephaly: Failure of cranial neural tube closure; incompatible with life
- Spina bifida: Failure of caudal neural tube closure; ranges from mild (spina bifida occulta) to severe (myelomeningocele)
- Encephalocele: Herniation of brain tissue through a defect in the skull
Folic acid supplementation before conception and during early pregnancy can reduce the risk of NTDs by approximately 70%.
6. Placental Development
The placenta is a temporary organ that develops during pregnancy to support the growing embryo and fetus. It serves as the interface between maternal and fetal circulations, facilitating essential functions such as nutrient exchange, gas exchange, waste elimination, hormone production, and immunological protection.
Placental development begins shortly after implantation and continues throughout the first trimester, with growth paralleling uterine development during the remainder of pregnancy.
6.1 Structure of the Placenta
The placenta is a disc-shaped organ that measures approximately 20 cm in diameter and 3 cm in thickness at term. It has both fetal and maternal components:
Fetal Component:
- Chorionic Plate: Forms the fetal surface of the placenta
- Chorionic Villi: Tree-like projections containing fetal blood vessels
- Umbilical Cord: Connects the fetus to the placenta
- Amnion: Innermost fetal membrane that forms the amniotic sac
Types of Chorionic Villi:
- Primary Villi: Cytotrophoblast covered by syncytiotrophoblast
- Secondary Villi: Primary villi plus mesenchymal core
- Tertiary Villi: Secondary villi with fetal blood vessels
Maternal Component:
- Decidua Basalis: Maternal tissue at the implantation site
- Intervillous Space: Contains maternal blood that bathes the chorionic villi
- Spiral Arteries: Modified maternal vessels that supply blood to the intervillous space
- Decidual Septa: Divide the placenta into 15-20 cotyledons
Placental Barrier:
Layers separating maternal and fetal blood (decrease as pregnancy advances):
- Syncytiotrophoblast
- Cytotrophoblast (diminishes by third trimester)
- Villous connective tissue (Wharton’s jelly)
- Fetal vascular endothelium
Key Point
Despite common misconceptions, maternal and fetal blood never normally mix in the placenta. They are separated by the placental barrier, which thins as pregnancy progresses to enhance exchange efficiency while maintaining separation. This barrier prevents direct contact between maternal and fetal blood cells but allows selective transport of gases, nutrients, waste products, and some other substances.
6.2 Function of the Placenta
The placenta performs numerous vital functions throughout pregnancy:
| Function | Description | Clinical Relevance |
|---|---|---|
| Gas Exchange | Oxygen diffuses from maternal to fetal circulation; carbon dioxide moves in the opposite direction | Placental insufficiency can lead to intrauterine growth restriction (IUGR) due to hypoxia |
| Nutrient Transfer | Glucose, amino acids, fatty acids, vitamins, and minerals cross from mother to fetus | Maternal malnutrition or impaired placental transport affects fetal growth |
| Waste Elimination | Urea, uric acid, and other metabolic wastes transfer from fetal to maternal circulation | Accumulation of waste products can be toxic to the developing fetus |
| Hormone Production | Produces hormones including hCG, estrogen, progesterone, human placental lactogen (hPL) | Hormonal assays are used to monitor placental function and pregnancy health |
| Immunological Protection | Prevents maternal immune rejection while allowing transfer of passive immunity (IgG antibodies) | Maternal-fetal incompatibility can lead to conditions like hemolytic disease of the newborn |
| Metabolism | Synthesizes glycogen, cholesterol, fatty acids; converts nutrients to forms usable by the fetus | Placental metabolic function is essential for proper fetal development |
Placental Transport Mechanisms:
- Simple Diffusion: Gases, water, urea, fatty acids
- Facilitated Diffusion: Glucose via GLUT transporters
- Active Transport: Amino acids, some ions
- Endocytosis/Exocytosis: IgG antibodies, lipoproteins
- Bulk Flow: Water following osmotic gradients
Placental Hormones:
- Human Chorionic Gonadotropin (hCG): Maintains corpus luteum; peaks at 8-10 weeks
- Progesterone: Maintains endometrium; produced by placenta after 8 weeks
- Estrogens: Promote uterine growth and mammary gland development
- Human Placental Lactogen (hPL): Modifies maternal metabolism to ensure fetal nutrition
- Corticotropin-Releasing Hormone (CRH): Involved in timing of parturition
Mnemonic: Placental Functions “NOURISH”
Nutrient transfer (glucose, amino acids, vitamins)
Oxygen exchange (and removal of carbon dioxide)
Urea and waste elimination
Reproductive hormone production (hCG, estrogen, progesterone)
Immune protection (barrier and IgG transfer)
Storage of glycogen and other nutrients
Hormone metabolism and conversion
Clinical Connection
Placental abnormalities can lead to serious complications during pregnancy:
- Placenta Previa: Placenta implants over or near the cervical os; causes painless vaginal bleeding
- Placental Abruption: Premature separation of the placenta from the uterine wall; painful bleeding
- Placenta Accreta/Increta/Percreta: Abnormal attachment of the placenta to the myometrium; risk of severe hemorrhage
- Placental Insufficiency: Reduced placental function leading to IUGR and fetal distress
7. Clinical Significance
Understanding embryological development and placental formation is crucial for nursing practice as it provides the foundation for comprehending normal and abnormal development, recognizing congenital anomalies, and providing appropriate care during pregnancy and the neonatal period.
Developmental Disorders by Stage:
- Fertilization/Cleavage Issues: Chromosomal disorders (Down syndrome, Turner syndrome)
- Implantation Abnormalities: Ectopic pregnancy, placenta previa
- Gastrulation Defects: Sirenomelia (fusion of lower limbs), caudal regression syndrome
- Neurulation Defects: Neural tube defects, microcephaly
- Placental Dysfunction: Preeclampsia, IUGR, placental insufficiency
Teratogen Exposure and Critical Periods:
Teratogens (agents that cause developmental abnormalities) affect different structures depending on the timing of exposure:
- Weeks 1-2: “All-or-none” effect (embryo either dies or recovers)
- Weeks 3-8: Major structural abnormalities (organogenesis period)
- Weeks 9-38: Functional defects and minor structural abnormalities
Common teratogens include certain medications (e.g., valproic acid, thalidomide), alcohol, tobacco, infectious agents (e.g., rubella, cytomegalovirus), and radiation.
Nursing Considerations:
- Preconception Counseling: Education about folic acid supplementation, avoiding teratogens, and genetic screening
- Prenatal Care: Monitoring for normal embryological development through appropriate screening and diagnostic tests
- High-Risk Pregnancy Identification: Recognizing factors that may compromise embryological or placental development
- Neonatal Assessment: Evaluating newborns for signs of abnormal development or birth defects
- Parent Education: Providing information about normal and abnormal development, potential complications, and management strategies
Clinical Case Study: Hydatidiform Mole
A hydatidiform mole is an abnormal pregnancy resulting from abnormal fertilization. In a complete mole, an empty egg (no maternal DNA) is fertilized by one or two sperm, resulting in paternal-only genetic material. The trophoblast develops excessively, but no embryo forms.
Presentation includes:
- Uterine enlargement greater than expected for gestational age
- Vaginal bleeding
- Extremely high hCG levels
- “Snowstorm” appearance on ultrasound
- No fetal heartbeat
This condition highlights the importance of understanding normal embryological development to recognize pathological processes.
8. Mnemonics for Embryological Development
Mnemonics are valuable tools for remembering complex embryological concepts and developmental sequences. Here’s a collection of useful mnemonics for nursing students:
Embryological Stages Sequence: “My Baby Grows Nicely”
Morula: Solid ball of cells
Blastocyst: Hollow ball with inner cell mass
Gastrulation: Formation of three germ layers
Neurulation: Formation of neural tube
Germ Layer Mnemonic: “Every Mom Endures”
Ectoderm: External (skin, nervous system)
Mesoderm: Middle (muscles, bones, blood)
Endoderm: Enteric (gut, respiratory tract)
Ectodermal Derivatives: “SEND”
Skin (epidermis and appendages)
External sense organs
Nervous system (central and peripheral)
Dental enamel and oral epithelium
Mesodermal Derivatives: “BOMB CURS”
Blood and blood vessels
Ovaries and testes
Muscles (all types)
Bones and cartilage
Connective tissue
Urinary system
Reproductive tract (except gonads)
Serous membranes
Endodermal Derivatives: “GILT”
Gastrointestinal tract epithelium
Internal glands (liver, pancreas, thyroid)
Lungs and respiratory tract epithelium
Tympanic cavity epithelium
Placental Functions: “PERFECT”
Provides nutrients
Excretes waste
Respiratory gas exchange
Filters harmful substances
Endocrine function
Circulatory connection
Transfers antibodies
Teratogen Susceptibility: “Critical Period Concept”
Remember that different organ systems have different critical periods of development when they are most susceptible to teratogens:
- Weeks 3-4: Heart, central nervous system
- Weeks 4-5: Upper limbs, eyes, ears
- Weeks 5-6: Lower limbs, palate
- Weeks 6-8: External genitalia
Mnemonic: “Hearts UEar Little Girls” (Heart, Upper Extremities, Lower Extremities, Genitalia)
9. Summary
Embryological development is a remarkably complex and precisely orchestrated process that transforms a single-celled zygote into a fully formed fetus. This journey involves multiple critical stages, each building upon the previous one to establish the foundation for human life.
Key Takeaways
- Pre-implantation Development: Fertilization initiates the embryological journey, followed by cleavage, morula formation, and blastocyst development.
- Implantation: The blastocyst embeds into the uterine endometrium, establishing the maternal-fetal interface essential for development.
- Gastrulation: Formation of the three primary germ layers—ectoderm, mesoderm, and endoderm—each giving rise to specific tissues and organs.
- Neurulation: Development of the neural tube from ectoderm, which will form the central nervous system, along with neural crest cell migration.
- Placental Development: Formation of the placenta from trophoblast cells and maternal decidua, creating an organ essential for fetal nutrition, gas exchange, waste elimination, hormone production, and immune protection.
Understanding the normal progression of embryological development provides the foundation for recognizing abnormal development and associated clinical conditions. For nursing students, this knowledge is invaluable for providing comprehensive care to pregnant women, assessing fetal well-being, identifying high-risk situations, and educating patients about normal development and potential complications.
The placenta, a remarkable temporary organ co-created by the mother and fetus, plays a crucial role in supporting embryological development throughout pregnancy. Its complex structure and diverse functions highlight the intricate relationship between maternal and fetal systems during gestation.
By mastering the concepts, sequences, and clinical implications of embryological development and placental formation, nursing students will be better prepared to provide evidence-based, compassionate care across the reproductive health spectrum.
© 2025 | Comprehensive Nursing Education Resources
