Types of Vials and Ampoules
Complete Guide for Nursing Students
Master the art of preparing injectable medicines with confidence and precision
Table of Contents
Introduction to Injectable Medicines
Precision Medicine
Injectable medicines provide precise dosing and rapid therapeutic action for critical patient care.
Rapid Action
Direct delivery to systemic circulation ensures immediate bioavailability and therapeutic response.
Safety First
Sterile preparation techniques prevent contamination and ensure patient safety in all clinical settings.
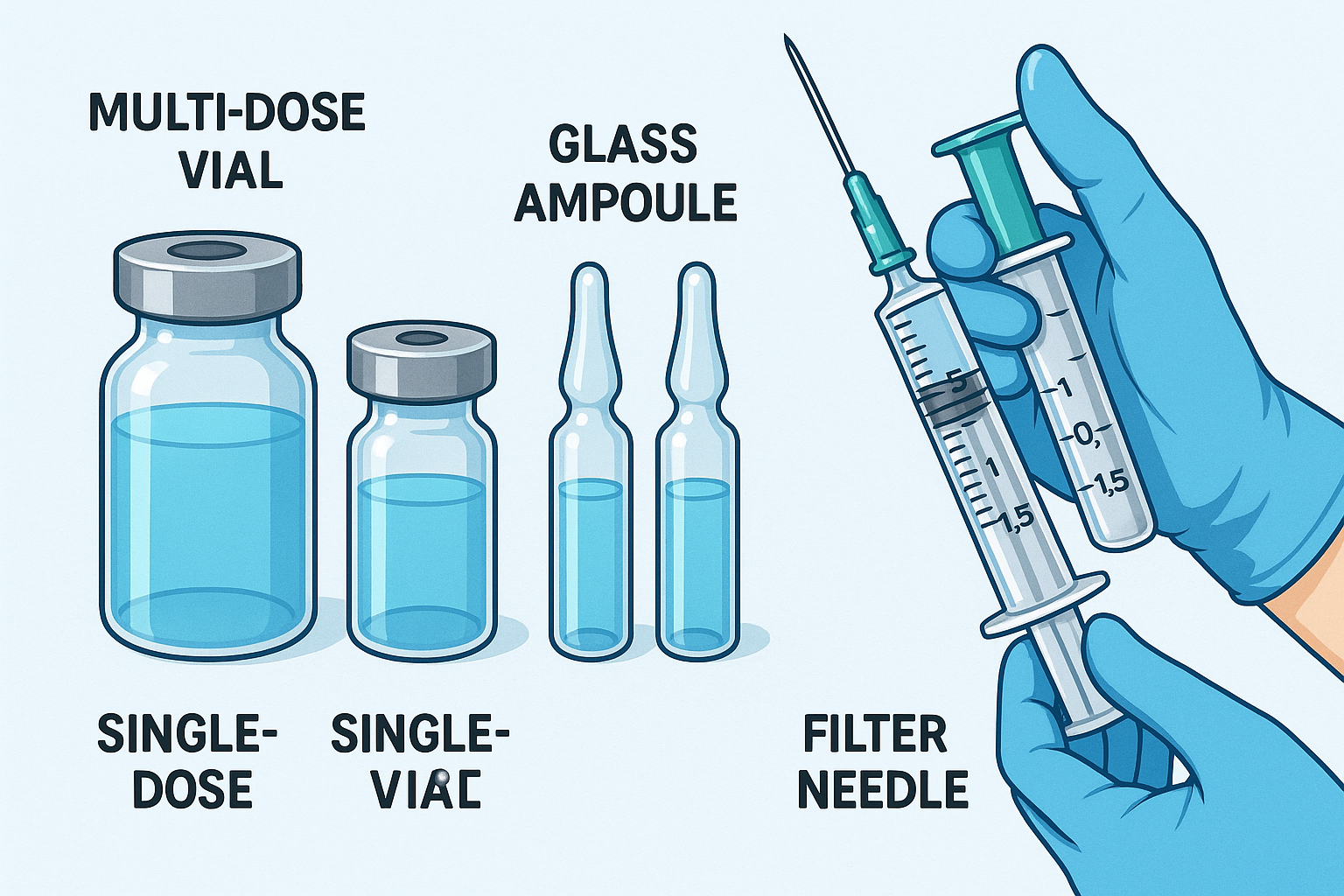
Educational illustration showing different types of vials and ampoules used in nursing practice
Injectable medicines represent one of the most critical aspects of modern healthcare delivery. As nursing professionals, understanding the proper handling, preparation, and administration of medications from vials and ampoules forms the cornerstone of safe patient care. These containers serve as the primary vessels for storing sterile pharmaceutical preparations that require parenteral administration.
The distinction between different container types directly impacts preparation techniques, storage requirements, and safety considerations. Multi-dose vials allow for multiple withdrawals while maintaining sterility, whereas single-dose containers prioritize immediate use with maximum safety. Understanding these fundamental differences enables nurses to make informed decisions about medication preparation protocols.
Memory Aid: VIALS Principle
- Verify – Always check medication name, dose, and expiration
- Inspect – Examine container for cracks, particles, or discoloration
- Aseptic – Maintain sterile technique throughout preparation
- Label – Properly identify prepared medications
- Safety – Follow institutional protocols and double-check procedures
Types of Vials
Vials represent the most commonly encountered medication containers in clinical practice. Understanding their various types, construction materials, and intended uses enables nurses to select appropriate preparation techniques and maintain medication integrity throughout the administration process.
| Vial Type | Capacity Range | Closure System | Primary Use | Key Features |
|---|---|---|---|---|
| Single-Dose Vials | 0.5-50 mL | Rubber stopper + aluminum seal | One-time use medications | No preservatives, immediate use required |
| Multi-Dose Vials | 10-100 mL | Self-sealing rubber stopper | Multiple withdrawals over time | Contains preservatives, 28-day use limit |
| Lyophilized Vials | 2-20 mL | Dual-chamber or reconstitution system | Freeze-dried medications | Requires reconstitution before use |
| Pre-filled Vials | 1-10 mL | Ready-to-use closure | Emergency medications | No preparation required, immediate use |
| Glass vs Plastic Vials | Variable | Material-specific sealing | Compatibility-based selection | Chemical compatibility considerations |
Single-Dose Vials
Single-dose vials contain medication intended for immediate, one-time use. These containers prioritize patient safety by eliminating the risk of cross-contamination between patients.
Key Characteristics:
- • Preservative-free formulations
- • Aluminum or plastic flip-off caps
- • Clear labeling for single-patient use
- • Sterile until first puncture
- • Must be discarded after initial access
Multi-Dose Vials
Multi-dose vials enable multiple withdrawals while maintaining sterility through self-sealing rubber stoppers and antimicrobial preservatives.
Key Characteristics:
- • Contains antimicrobial preservatives
- • Self-sealing rubber stopper design
- • 28-day use limitation after first puncture
- • Requires proper storage conditions
- • Date marking mandatory after opening
Critical Safety Considerations for Vials
Never Mix Vial Types:
- • Single-dose vials cannot be stored for later use
- • Multi-dose vials require preservative compatibility
- • Cross-contamination risks increase with improper handling
Storage Requirements:
- • Temperature-controlled environments essential
- • Light-sensitive medications require protection
- • Proper orientation prevents stopper degradation
Types of Ampoules
Ampoules represent the gold standard for sterile, single-dose medication storage. These sealed glass containers provide maximum protection against contamination while ensuring medication stability throughout extended storage periods. Understanding ampoule varieties and their specific applications enhances preparation efficiency and patient safety.
Standard Glass Ampoules
- Capacity: 0.5-20 mL
- Break System: Score line or color band
- Material: Borosilicate glass
- Use: Single-dose medications
- Advantage: Complete sterility assurance
Amber Glass Ampoules
- Capacity: 1-10 mL
- Protection: UV light filtration
- Material: Tinted borosilicate glass
- Use: Light-sensitive medications
- Advantage: Photodegradation prevention
Easy-Break Ampoules
- Capacity: 1-5 mL
- Design: Pre-scored neck
- Safety: Reduced injury risk
- Use: Frequent-access medications
- Advantage: Enhanced healthcare worker safety
Ampoule Construction and Design Principles
Ampoules utilize sophisticated engineering principles to maintain sterility and medication integrity. The hermetic seal created during manufacturing eliminates air exchange, preventing oxidation and contamination. Glass composition varies based on medication compatibility requirements, with Type I borosilicate glass providing superior chemical resistance for reactive compounds.
Ampoule Advantages
- • Complete sterility maintenance until opening
- • Zero preservative requirements
- • Extended shelf life stability
- • Tamper-evident design
- • Chemical inertness of glass
- • Precise dosing accuracy
- • Environmental temperature stability
Ampoule Limitations
- • Single-use restriction
- • Glass particle contamination risk
- • Breaking technique requirements
- • Sharp edge injury potential
- • Cannot be resealed
- • Requires filter needle use
- • Storage space considerations
Memory Aid: AMPOULE Safety
- Always inspect for cracks or damage before use
- Mark the break point clearly before opening
- Protect hands with gauze or alcohol swab
- Open away from body and other personnel
- Use filter needle to prevent glass particles
- Label prepared medication immediately
- Eliminate broken ampoule safely in sharps container
Preparation Techniques
Mastering proper preparation techniques for medications from vials and ampoules requires understanding both theoretical principles and practical skills. These techniques form the foundation of safe medication administration and directly impact patient outcomes through contamination prevention and dosing accuracy.
Vial Preparation Technique
Step 1: Pre-preparation Assessment
- • Verify medication order against container label
- • Check expiration date and lot number
- • Inspect vial for cracks, chips, or contamination
- • Confirm appropriate storage conditions maintained
- • Gather necessary supplies and equipment
Step 2: Sterile Field Preparation
- • Establish clean work surface
- • Perform hand hygiene according to protocol
- • Don appropriate personal protective equipment
- • Arrange supplies within sterile field
- • Remove vial cap and inspect rubber stopper
Step 3: Surface Disinfection
- • Clean rubber stopper with 70% isopropyl alcohol
- • Allow minimum 30-second contact time
- • Permit complete air drying
- • Avoid touching cleaned surface
- • Maintain sterile technique throughout process
Step 4: Needle Selection and Insertion
- • Select appropriate needle gauge (18-21G typical)
- • Insert needle at 90-degree angle
- • Penetrate stopper center to minimize coring
- • Advance needle completely through stopper
- • Maintain single, smooth insertion motion
Step 5: Medication Withdrawal
- • Inject air volume equal to medication volume needed
- • Invert vial and maintain needle in solution
- • Withdraw prescribed medication amount slowly
- • Tap syringe to dislodge air bubbles
- • Expel excess air while maintaining sterility
Step 6: Final Verification and Labeling
- • Verify accurate medication volume in syringe
- • Remove needle from vial using smooth motion
- • Replace needle with appropriate administration needle
- • Label syringe with medication details
- • Record vial opening time if multi-dose
Ampoule Preparation Technique
Pre-Breaking Assessment
- • Inspect ampoule for cracks or damage
- • Verify medication clarity and color
- • Locate score line or break point
- • Check for precipitates or particles
- • Confirm medication identification
Safe Breaking Technique
- • Tap ampoule to settle medication in base
- • Wrap neck with gauze or alcohol swab
- • Hold ampoule away from body
- • Break with quick, snapping motion
- • Dispose of ampoule top in sharps container
Filtered Withdrawal
- • Attach filter needle to syringe
- • Insert needle into ampoule solution
- • Withdraw medication slowly and steadily
- • Replace filter needle with administration needle
- • Expel air and verify dose accuracy
Critical Filter Needle Requirements
Filter needles are mandatory when withdrawing medication from ampoules to prevent glass particle administration. These specialized needles contain a 5-micron filter that traps microscopic glass fragments while allowing medication passage.
Filter Specifications:
- • 5-micron filtration capacity minimum
- • Single-use application only
- • Compatible with standard syringe connections
- • Available in various gauge sizes
Usage Protocols:
- • Never use for patient administration
- • Replace with standard needle before injection
- • Maintain sterility throughout procedure
- • Document filter needle usage in records
Advanced Preparation Scenarios
Reconstitution Procedures
Lyophilized medications require careful reconstitution to maintain therapeutic potency and prevent foaming.
- • Use recommended diluent type and volume
- • Inject diluent slowly along vial wall
- • Swirl gently to dissolve powder completely
- • Allow settling time to eliminate foam
- • Verify complete dissolution before withdrawal
Medication Compatibility
Multiple medication combinations require compatibility verification to prevent precipitation or inactivation.
- • Consult compatibility charts before mixing
- • Verify pH compatibility ranges
- • Check for visible precipitation formation
- • Consider administration timing requirements
- • Document all medication combinations used
Safety Protocols
Comprehensive safety protocols form the backbone of secure medication preparation from vials and ampoules. These evidence-based guidelines protect both healthcare providers and patients while ensuring medication integrity throughout the preparation and administration process.
Personal Protection
- Hand Protection: Nitrile gloves, double-gloving for hazardous drugs
- Eye Protection: Safety glasses or face shields
- Respiratory Protection: N95 masks for aerosolized medications
- Body Protection: Fluid-resistant gowns or aprons
- Foot Protection: Closed-toe shoes with fluid resistance
Environmental Controls
- Laminar Flow: Biological safety cabinets for hazardous drugs
- Clean Rooms: ISO-classified environments for sterile compounding
- Air Filtration: HEPA filtration systems
- Pressure Gradients: Negative pressure for containment
- Surface Disinfection: Regular cleaning protocols
Process Verification
- Double-Check System: Independent verification by second nurse
- Barcode Scanning: Electronic verification systems
- Documentation: Complete preparation records
- Labeling: Clear, accurate medication labels
- Time Limits: Preparation-to-administration timeframes
Contamination Prevention Strategies
Microbial Contamination Prevention
Aseptic Technique Principles:
- • Maintain sterile field integrity throughout procedure
- • Use sterile supplies and equipment exclusively
- • Minimize airborne particle exposure time
- • Avoid touching sterile surfaces with non-sterile items
- • Perform hand hygiene at critical contamination points
Critical Control Points:
- • Vial stopper and ampoule neck disinfection
- • Needle and syringe sterility maintenance
- • Work surface cleaning and preparation
- • Air quality monitoring and control
- • Personnel movement restriction in sterile areas
Cross-Contamination Prevention
Patient-to-Patient:
- • Single-use vials mandatory
- • Dedicated syringes per patient
- • Individual medication preparation
- • Separate storage systems
Medication-to-Medication:
- • Separate preparation areas
- • Clean syringes between medications
- • Dedicated mixing equipment
- • Sequential preparation protocols
Environment-to-Product:
- • Controlled access areas
- • Regular surface disinfection
- • Air filtration maintenance
- • Personnel hygiene protocols
Healthcare Worker Injury Prevention
Needlestick Injury Prevention
- • Use safety-engineered needles with automatic retraction
- • Never recap needles using two-handed technique
- • Dispose of sharps immediately after use
- • Maintain sharps containers at point of use
- • Replace sharps containers when three-quarters full
- • Report all needlestick injuries immediately
- • Follow post-exposure prophylaxis protocols
Glass Injury Prevention
- • Wrap ampoule necks with protective material
- • Break ampoules away from body and others
- • Use proper breaking technique with single motion
- • Dispose of broken glass in puncture-proof containers
- • Inspect hands for cuts after ampoule handling
- • Use appropriate first aid for minor cuts
- • Seek immediate medical attention for deep lacerations
Memory Aid: SAFETY Protocol
- Sterile technique maintained throughout entire process
- Appropriate personal protective equipment utilized
- Filter needles used for all ampoule withdrawals
- Environment controlled for contamination prevention
- Time limits observed for preparation stability
- Yield verification through double-check systems
Common Errors & Prevention
Understanding common preparation errors and their prevention strategies significantly reduces medication administration risks. These evidence-based insights help nursing students develop error-prevention mindsets and implement systematic safeguards in clinical practice.
High-Risk Error Categories
Dosage Calculation Errors
Frequency: 23% of medication errors
- • Decimal point placement mistakes
- • Unit conversion errors
- • Concentration calculation failures
- • Pediatric dosing miscalculations
Contamination Events
Frequency: 18% of preparation errors
- • Sterile technique breaches
- • Multi-dose vial contamination
- • Cross-contamination between patients
- • Environmental contamination exposure
Identification Errors
Frequency: 16% of preparation errors
- • Wrong medication selection
- • Patient identification failures
- • Look-alike medication confusion
- • Concentration strength errors
Prevention Strategies
Calculation Verification
- • Use electronic calculation aids
- • Implement independent double-checks
- • Standardize calculation protocols
- • Provide decimal point verification tools
- • Use unit-dose preparations when available
Contamination Prevention
- • Enforce strict aseptic technique training
- • Use single-dose vials when possible
- • Implement environmental monitoring
- • Provide adequate preparation space
- • Use barrier protection systems
Identification Accuracy
- • Implement barcode scanning systems
- • Use tall-man lettering for similar drugs
- • Separate look-alike medications
- • Require two-person verification
- • Use automated dispensing systems
Specific Error Scenarios and Solutions
| Error Type | Common Scenario | Potential Consequence | Prevention Strategy |
|---|---|---|---|
| Needle Gauge Error | Using 25G needle for viscous medication | Incomplete withdrawal, dosing error | Match needle gauge to medication viscosity |
| Vial Type Confusion | Storing single-dose vial for later use | Contamination, patient infection | Clear labeling and education protocols |
| Air Bubble Retention | Failing to expel air from syringe | Air embolism, incorrect dosing | Systematic air removal procedures |
| Filter Needle Omission | Direct withdrawal from ampoule without filter | Glass particle injection | Mandatory filter needle protocols |
| Reconstitution Error | Wrong diluent volume or type | Medication inactivation, toxicity | Standardized reconstitution guidelines |
Error Reporting and Continuous Improvement
Effective error prevention requires systematic reporting and analysis of preparation errors to identify patterns and implement targeted improvements.
Reporting Elements:
- • Error type and contributing factors
- • Environmental conditions
- • Personnel experience level
- • Equipment or system failures
- • Patient outcome impacts
Analysis Methods:
- • Root cause analysis
- • Failure mode analysis
- • Statistical trend identification
- • Comparative benchmarking
- • Human factors assessment
Improvement Actions:
- • Protocol standardization
- • Additional staff training
- • Technology implementation
- • Environmental modifications
- • Quality assurance programs
Clinical Applications
Clinical applications of vials and ampoules span diverse healthcare settings, each with unique requirements for medication preparation and safety protocols. Understanding these specific contexts enhances nursing competency and patient care outcomes across different practice environments.
Emergency Department
- Priority: Rapid medication preparation
- Common Types: Pre-filled syringes, emergency vials
- Challenges: High-stress environment, time constraints
- Safety Focus: Quick verification protocols
- Typical Medications: Epinephrine, atropine, naloxone
Operating Room
- Priority: Sterile technique maintenance
- Common Types: Single-dose vials, ampoules
- Challenges: Sterile field requirements
- Safety Focus: Contamination prevention
- Typical Medications: Anesthetics, muscle relaxants
Pediatric Unit
- Priority: Precise micro-dosing
- Common Types: Small-volume vials, dilutions
- Challenges: Weight-based calculations
- Safety Focus: Dosing accuracy verification
- Typical Medications: Antibiotics, vaccines
Specialized Clinical Applications
Chemotherapy Preparation
Hazardous drug preparation requires specialized equipment and enhanced safety protocols using both vials and ampoules.
Critical Care Applications
Intensive care units require continuous medication preparation with emphasis on compatibility and stability.
Quality Assurance Programs
Comprehensive quality assurance programs ensure consistent medication preparation standards across all clinical applications.
Competency Assessment
Regular skills validation and training programs
Process Monitoring
Continuous observation and documentation
Outcome Analysis
Statistical review of preparation accuracy
Process Improvement
Systematic enhancement implementation
Technology Integration in Clinical Practice
| Technology | Application | Benefits | Implementation Considerations |
|---|---|---|---|
| Barcode Scanning | Medication verification at preparation | Reduced identification errors, improved accuracy | Staff training, system integration requirements |
| Automated Dispensing | Controlled access to vials and ampoules | Enhanced security, usage tracking | Initial investment, maintenance protocols |
| Smart Pumps | Calculation assistance and safety limits | Reduced dosing errors, protocol compliance | Drug library maintenance, user interface training |
| Electronic Records | Digital documentation and tracking | Complete audit trails, data analysis | System integration, data security measures |
Global Best Practices
Healthcare systems worldwide have developed innovative approaches to vials and ampoules management, creating evidence-based practices that enhance patient safety and improve clinical outcomes. These international perspectives provide valuable insights for advancing nursing practice standards.
European Union Standards
The European Medicines Agency (EMA) has established comprehensive guidelines for injectable medication preparation and handling.
- GMP Requirements: Good Manufacturing Practice compliance for all preparation facilities
- Qualification Standards: Mandatory personnel certification for sterile compounding
- Environmental Monitoring: Continuous air quality and surface contamination assessment
- Traceability Systems: Complete batch tracking from manufacture to administration
- Quality Control: Regular sterility testing and endotoxin monitoring
Canadian Healthcare Excellence
Health Canada promotes integrated safety systems combining technology and human factors approaches.
- ISMP Canada: Institute for Safe Medication Practices leadership in error prevention
- Technology Integration: Widespread adoption of smart infusion systems
- Interprofessional Collaboration: Pharmacist-nurse partnership in preparation protocols
- Continuous Education: Mandatory ongoing competency development programs
- Error Reporting Culture: Non-punitive incident reporting and learning systems
Regional Healthcare Innovations
Japanese Precision Systems
Japan’s healthcare system emphasizes precision engineering and quality control in medication preparation.
- • Automated medication preparation robots
- • Advanced quality control testing
- • Standardized preparation protocols
- • Continuous improvement methodologies
- • Integration of artificial intelligence
Australian Safety Culture
Australia’s Therapeutic Goods Administration promotes comprehensive safety cultures in healthcare facilities.
- • National medication safety programs
- • Standardized competency assessments
- • Inter-facility learning networks
- • Technology-enhanced verification systems
- • Patient engagement in safety initiatives
Scandinavian Excellence
Nordic countries demonstrate leadership in healthcare technology integration and patient safety initiatives.
- • Electronic health record integration
- • Evidence-based practice protocols
- • Comprehensive staff development programs
- • Patient safety outcome monitoring
- • International collaboration networks
World Health Organization (WHO) Guidelines
The WHO provides global leadership in establishing international standards for injectable medication safety and preparation protocols.
Core WHO Recommendations:
- • Universal Precautions: Standard infection control measures for all patient interactions
- • Safe Injection Practices: One needle, one syringe, one vial per patient principle
- • Healthcare Worker Protection: Comprehensive safety equipment and training programs
- • Quality Assurance: Systematic monitoring and improvement processes
- • Education Standards: Evidence-based curriculum development for healthcare professionals
Global Implementation Strategies:
- • Resource Adaptation: Scalable practices for diverse healthcare settings
- • Cultural Sensitivity: Integration with local healthcare practices and beliefs
- • Technology Transfer: Knowledge sharing between developed and developing nations
- • Outcome Measurement: Standardized metrics for international comparison
- • Continuous Learning: Global networks for sharing best practices and innovations
Future Directions in Global Practice
Emerging Technologies
- • Artificial Intelligence: Predictive algorithms for medication preparation optimization
- • Robotics: Automated preparation systems with enhanced precision
- • Internet of Things: Smart monitoring systems for environmental conditions
- • Blockchain Technology: Immutable medication tracking and verification systems
- • Augmented Reality: Enhanced visualization for complex preparation procedures
Global Collaboration Initiatives
- • International Standards: Harmonized global protocols for medication preparation
- • Knowledge Exchange: Cross-border learning and best practice sharing
- • Research Collaboration: Multi-national studies on medication safety outcomes
- • Technology Development: Joint innovation projects for healthcare advancement
- • Education Partnerships: International nursing curriculum development initiatives
Global Best Practice Implementation: WORLDWIDE
- World Health Organization guidelines compliance and integration
- Organizational commitment to continuous safety improvement
- Research-based evidence integration in clinical protocols
- Leadership engagement in safety culture development
- Data-driven decision making for practice enhancement
- Workforce development through ongoing education programs
- International collaboration and knowledge sharing
- Development of innovative solutions for emerging challenges
- Evaluation and continuous improvement of all processes
Conclusion
Mastering the preparation of injectable medicines from vials and ampoules represents a fundamental competency for nursing professionals. Through understanding container types, implementing proper techniques, and maintaining rigorous safety protocols, nurses ensure optimal patient outcomes while protecting themselves and colleagues from preventable harm.
Safety First
Always prioritize patient and provider safety through rigorous adherence to established protocols.
Continuous Learning
Stay updated with emerging best practices and technological advances in medication preparation.
Collaborative Excellence
Work with interdisciplinary teams to implement and improve medication safety initiatives.
