Immunochemistry for Nursing Students
Structure & Functions of Immunoglobulins | ELISA Investigations
Introduction to Immunochemistry
Immunochemistry is the branch of chemistry that deals with the chemical basis of immunological phenomena. For nursing professionals, understanding immunochemistry is crucial for patient care, diagnostic interpretation, and therapeutic interventions. This comprehensive guide focuses on immunoglobulins (antibodies) and their clinical applications through ELISA testing.
Why This Matters for Nurses
Understanding immunochemistry enables nurses to better interpret laboratory results, recognize immune-related disorders, administer immunoglobulin therapies safely, and educate patients about their immune status and treatment options.
Structure of Immunoglobulins
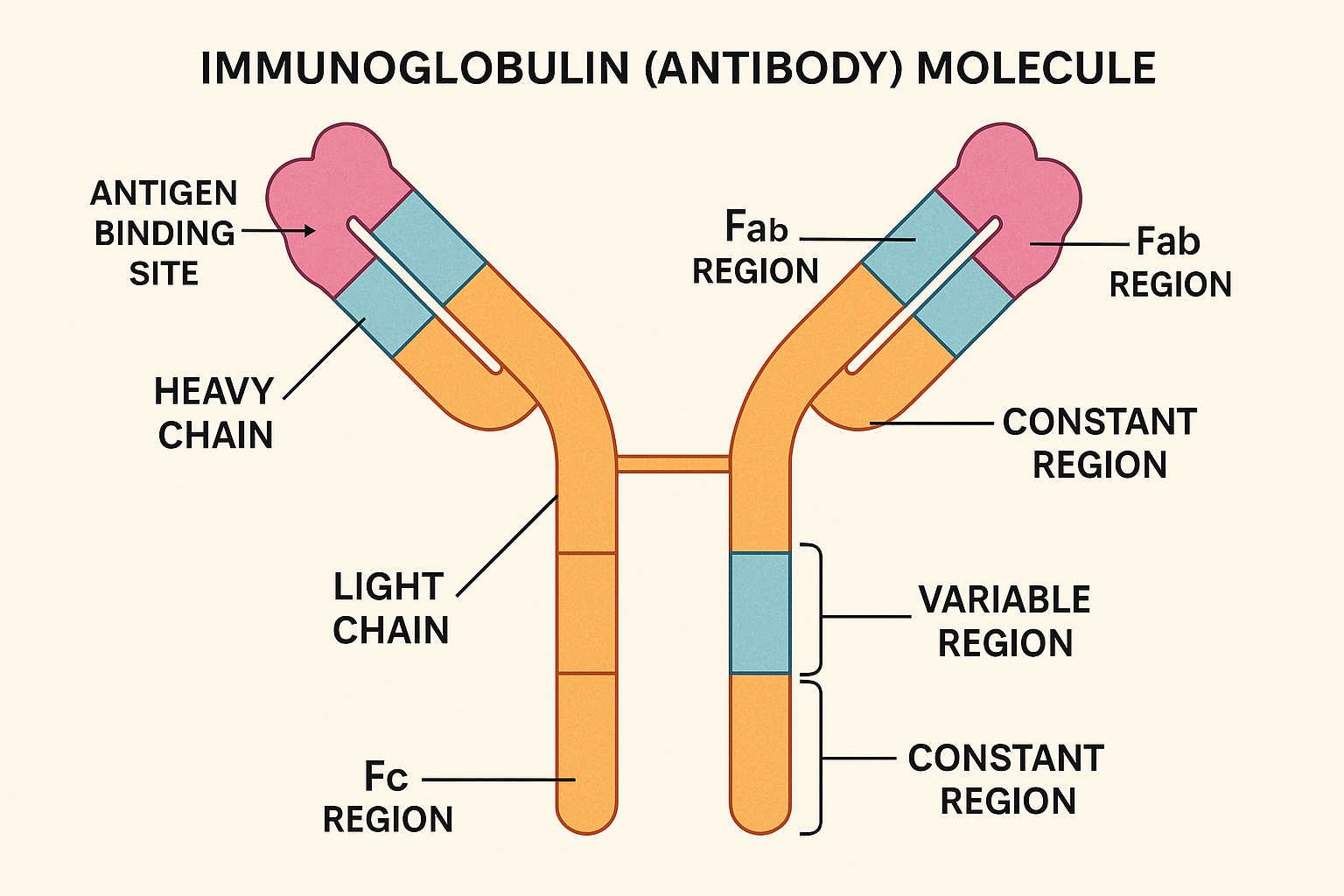
Figure 1: Detailed structure of an immunoglobulin (antibody) molecule showing all major components
Basic Structure Components
Heavy Chains
- Two identical heavy chains
- Molecular weight: ~50-70 kDa
- Contains variable (VH) and constant (CH) regions
- Determines antibody class (isotype)
Light Chains
- Two identical light chains
- Molecular weight: ~25 kDa
- Contains variable (VL) and constant (CL) regions
- Two types: Kappa (κ) and Lambda (λ)
Memory Aid: “Y” Structure
Y-shaped Antibody:
Y = Two arms (Fab regions) + One stem (Fc region)
Fab = Fragment antigen binding (Variable regions)
Fc = Fragment crystallizable (Constant region)
Functional Regions
Fab Region (Antigen Binding)
- Location: Arms of the Y-structure
- Composition: Variable regions of both heavy and light chains
- Function: Specific antigen recognition and binding
- Features: Contains complementarity-determining regions (CDRs)
- Clinical significance: Determines antibody specificity
Fc Region (Effector Function)
- Location: Stem of the Y-structure
- Composition: Constant regions of heavy chains
- Function: Effector functions (complement activation, cell binding)
- Features: Determines biological activity
- Clinical significance: Target for therapeutic antibodies
Key Clinical Point
The hinge region between Fab and Fc allows flexibility in antigen binding while maintaining effector function. This flexibility is crucial for cross-linking antigens and activating complement cascade.
Classes of Immunoglobulins
| Class | Structure | Location | Function | Normal Range | Clinical Significance |
|---|---|---|---|---|---|
| IgG | Monomer | Serum, tissues | Long-term immunity, placental transfer | 7-16 g/L | Most abundant; indicates past infection |
| IgM | Pentamer | Serum, lymph | First response, complement activation | 0.4-2.3 g/L | Acute infection marker |
| IgA | Monomer/Dimer | Secretions, mucosal surfaces | Mucosal immunity | 0.7-4.0 g/L | Deficiency causes recurrent infections |
| IgE | Monomer | Tissues, mast cells | Allergic reactions, parasitic infections | 0-0.4 g/L | Elevated in allergies and parasites |
| IgD | Monomer | B cell surface | B cell activation | 0-0.4 g/L | Marker of B cell maturation |
Memory Aid: “GAMED”
Good – IgG (Most abundant, Good for long-term immunity)
Access – IgA (Accesses mucosal surfaces)
Major – IgM (Major first responder)
Enemy – IgE (Enemy of allergens)
Developer – IgD (Develops B cells)
Functions of Immunoglobulins
Antigen Recognition
- • Specific binding to antigens
- • Neutralization of pathogens
- • Prevention of cellular invasion
- • Toxin neutralization
Complement Activation
- • Classical pathway activation
- • Cell lysis and death
- • Opsonization enhancement
- • Inflammatory response
Opsonization
- • Marking pathogens for destruction
- • Enhanced phagocytosis
- • Macrophage activation
- • Pathogen clearance
ADCC Function
- • Antibody-dependent cellular cytotoxicity
- • NK cell activation
- • Targeted cell killing
- • Tumor surveillance
Passive Immunity
- • Maternal antibody transfer
- • Placental transport (IgG)
- • Breast milk antibodies (IgA)
- • Newborn protection
Allergic Reactions
- • IgE-mediated hypersensitivity
- • Mast cell degranulation
- • Histamine release
- • Immediate hypersensitivity
Clinical Applications
Understanding immunoglobulin functions helps nurses recognize immune deficiencies, autoimmune disorders, and allergic reactions. This knowledge is essential for patient assessment, medication administration, and patient education.
ELISA: Enzyme-Linked Immunosorbent Assay
What is ELISA?
ELISA is a plate-based assay technique used to detect and quantify specific proteins, antibodies, or antigens in biological samples. It combines the specificity of antibodies with the sensitivity of enzyme detection systems.
Types of ELISA
Direct ELISA
- Principle: Antigen coated on plate
- Detection: Enzyme-labeled primary antibody
- Advantages: Simple, fast, less background
- Disadvantages: Less sensitive, expensive
- Use: Antigen detection, screening
Indirect ELISA
- Principle: Antigen coated on plate
- Detection: Primary + enzyme-labeled secondary antibody
- Advantages: More sensitive, versatile
- Disadvantages: More complex, potential cross-reactivity
- Use: Antibody detection, serology
Sandwich ELISA
- Principle: Capture antibody coated on plate
- Detection: Antigen captured between two antibodies
- Advantages: Highly specific, quantitative
- Disadvantages: Requires two specific antibodies
- Use: Protein quantification, diagnostics
Competitive ELISA
- Principle: Competition between sample and labeled antigen
- Detection: Inverse relationship with concentration
- Advantages: Works with small molecules
- Disadvantages: Less intuitive, requires optimization
- Use: Drug monitoring, small molecule detection
ELISA Procedure
Step 1: Coating
Antigen or antibody immobilized on microplate wells
Step 2: Blocking
Non-specific binding sites blocked with protein solution
Step 3: Sample Addition
Patient sample added to wells for specific binding
Step 4: Detection
Enzyme-labeled antibody added for detection
Step 5: Substrate Addition
Chromogenic substrate produces color change
Step 6: Reading
Optical density measured at specific wavelength
Memory Aid: “CBSDSM”
Coat – Coat the plate with antigen/antibody
Block – Block non-specific binding sites
Sample – Add patient sample
Detect – Add detection antibody
Substrate – Add enzyme substrate
Measure – Measure optical density
ELISA Interpretation & Clinical Significance
Result Interpretation
Positive Results
- Indicates: Presence of target analyte
- Color: Intense color development
- OD Value: Above cutoff threshold
- Clinical meaning: Active infection, immunity, or disease
- Nursing action: Report to physician, monitor patient
Negative Results
- Indicates: Absence of target analyte
- Color: Minimal or no color development
- OD Value: Below cutoff threshold
- Clinical meaning: No infection, no immunity, or normal
- Nursing action: Document results, consider prevention
Common Clinical Applications
| Test | Target | Clinical Use | Positive Result Meaning | Nursing Considerations |
|---|---|---|---|---|
| HIV ELISA | HIV antibodies | HIV screening | HIV infection (requires confirmation) | Confidentiality, counseling, follow-up |
| Hepatitis B | HBsAg, HBsAb | Hepatitis B status | Active infection or immunity | Isolation precautions, vaccination status |
| COVID-19 | SARS-CoV-2 antibodies | Past infection, immunity | Previous exposure or vaccination | Infection control, immunity assessment |
| Pregnancy Test | hCG hormone | Pregnancy detection | Pregnancy confirmed | Prenatal care, medication safety |
| Autoimmune | Autoantibodies | Autoimmune disease | Autoimmune process active | Disease monitoring, treatment response |
Important Clinical Considerations
- • False Positives: Cross-reactivity, autoimmune conditions, recent vaccination
- • False Negatives: Early infection, immunocompromised state, technical errors
- • Confirmation Testing: Positive screening tests often require confirmatory testing
- • Window Period: Time between infection and antibody detection
Nursing Implementation in Immunochemistry
Pre-Test Responsibilities
- Patient Education: Explain test purpose, procedure, and expectations
- Informed Consent: Obtain consent for specific tests (HIV, genetic testing)
- Medication Review: Identify medications that may affect results
- Fasting Requirements: Ensure proper preparation if required
- Patient Anxiety: Address concerns and provide emotional support
Sample Collection
- Proper Technique: Use aseptic technique for blood draws
- Correct Tubes: Use appropriate collection tubes (serum vs. plasma)
- Sample Handling: Proper storage and transport to laboratory
- Chain of Custody: Maintain proper documentation for legal tests
- Patient Comfort: Minimize discomfort during collection
Result Interpretation & Communication
- Critical Values: Immediately report critical results to physician
- Patient Communication: Explain results in understandable terms
- Privacy Protection: Maintain confidentiality of sensitive results
- Follow-up Care: Coordinate additional testing or referrals
- Documentation: Accurately document results and patient responses
Therapeutic Applications
Immunoglobulin Therapy
- IVIG Administration: Intravenous immunoglobulin for immunodeficiency
- Monitoring: Watch for infusion reactions and side effects
- Dosing: Calculate appropriate doses based on patient weight
- Indications: Primary immunodeficiency, autoimmune diseases
- Complications: Hemolysis, thrombosis, kidney injury
Monoclonal Antibodies
- Cancer Therapy: Targeted treatment for specific cancers
- Autoimmune Disorders: TNF inhibitors, IL-6 blockers
- Infection Control: COVID-19 monoclonal antibodies
- Infusion Reactions: Monitor for hypersensitivity reactions
- Immunosuppression: Increased infection risk
Clinical Scenarios for Nurses
Scenario 1: Immunodeficiency
Patient with recurrent infections, low IgG levels
- • Assess infection history and patterns
- • Monitor IVIG therapy effectiveness
- • Educate about infection prevention
- • Coordinate with immunologist
Scenario 2: Autoimmune Disease
Patient with elevated autoantibodies, joint pain
- • Monitor disease activity markers
- • Assess treatment response
- • Provide symptom management
- • Educate about disease process
Quality Control in Immunochemistry
Pre-Analytical Phase
- • Proper sample collection
- • Correct patient identification
- • Appropriate storage conditions
- • Timely transport to lab
- • Sample integrity assessment
Analytical Phase
- • Calibration standards
- • Quality control samples
- • Reagent validation
- • Equipment maintenance
- • Proficiency testing
Post-Analytical Phase
- • Result verification
- • Critical value reporting
- • Proper documentation
- • Result communication
- • Follow-up coordination
Common Sources of Error
Pre-Analytical Errors
- • Improper patient preparation
- • Sample contamination
- • Incorrect collection tubes
- • Hemolysis or lipemia
- • Delayed processing
Analytical Errors
- • Reagent degradation
- • Equipment malfunction
- • Inadequate washing
- • Cross-contamination
- • Timing errors
Future Developments in Immunochemistry
Technological Advances
- • Microfluidic devices for point-of-care testing
- • Automated high-throughput systems
- • Digital ELISA for ultra-sensitive detection
- • Multiplex assays for simultaneous testing
- • Smartphone-based detection systems
Personalized Medicine
- • Pharmacogenomic testing
- • Biomarker-guided therapy
- • Precision immunotherapy
- • Companion diagnostics
- • Predictive testing algorithms
Implications for Nursing Practice
Future developments will require nurses to adapt to new technologies, understand complex test interpretations, and provide advanced patient education. Continuous professional development will be essential to keep pace with evolving immunochemical testing methodologies.
Summary & Key Takeaways
Immunoglobulin Structure
- • Y-shaped molecules with Fab and Fc regions
- • Heavy and light chains with variable and constant regions
- • Five classes: IgG, IgM, IgA, IgE, IgD
- • Specific functions for immune protection
ELISA Applications
- • Sensitive and specific detection method
- • Multiple formats for different applications
- • Wide clinical use in diagnostics
- • Requires proper interpretation and follow-up
Final Memory Aid: “IMMUNE”
Immunoglobulins protect the body
Multiple classes serve different functions
Monitoring through ELISA testing
Understanding results guides treatment
Nursing care ensures patient safety
Education empowers patients and families
