🥞 Pancreatic Tumors: Complete Nursing Guide
Essential Knowledge for Nursing Students & Healthcare Professionals
🎯 Overview & Epidemiology
Pancreatic tumors represent one of the most challenging malignancies in modern medicine, with pancreatic adenocarcinoma being the most common and aggressive form. These tumors arise from the pancreatic ductal epithelium and are notorious for their poor prognosis and late-stage presentation. As nursing professionals, understanding the complexity of pancreatic tumors is crucial for providing comprehensive patient care and support.
📊 Key Statistics
- Incidence: 4th leading cause of cancer-related deaths globally
- 5-year survival rate: Approximately 10-12%
- Peak incidence: 60-70 years of age
- Gender distribution: Slightly higher in males (1.3:1 ratio)
- Geographic variation: Higher rates in developed countries
The aggressive nature of pancreatic tumors stems from their tendency to metastasize early, often before symptoms become apparent. This silent progression makes early detection extremely challenging, emphasizing the importance of recognizing subtle clinical signs and implementing comprehensive nursing care strategies.
🫀 Pancreatic Anatomy & Function
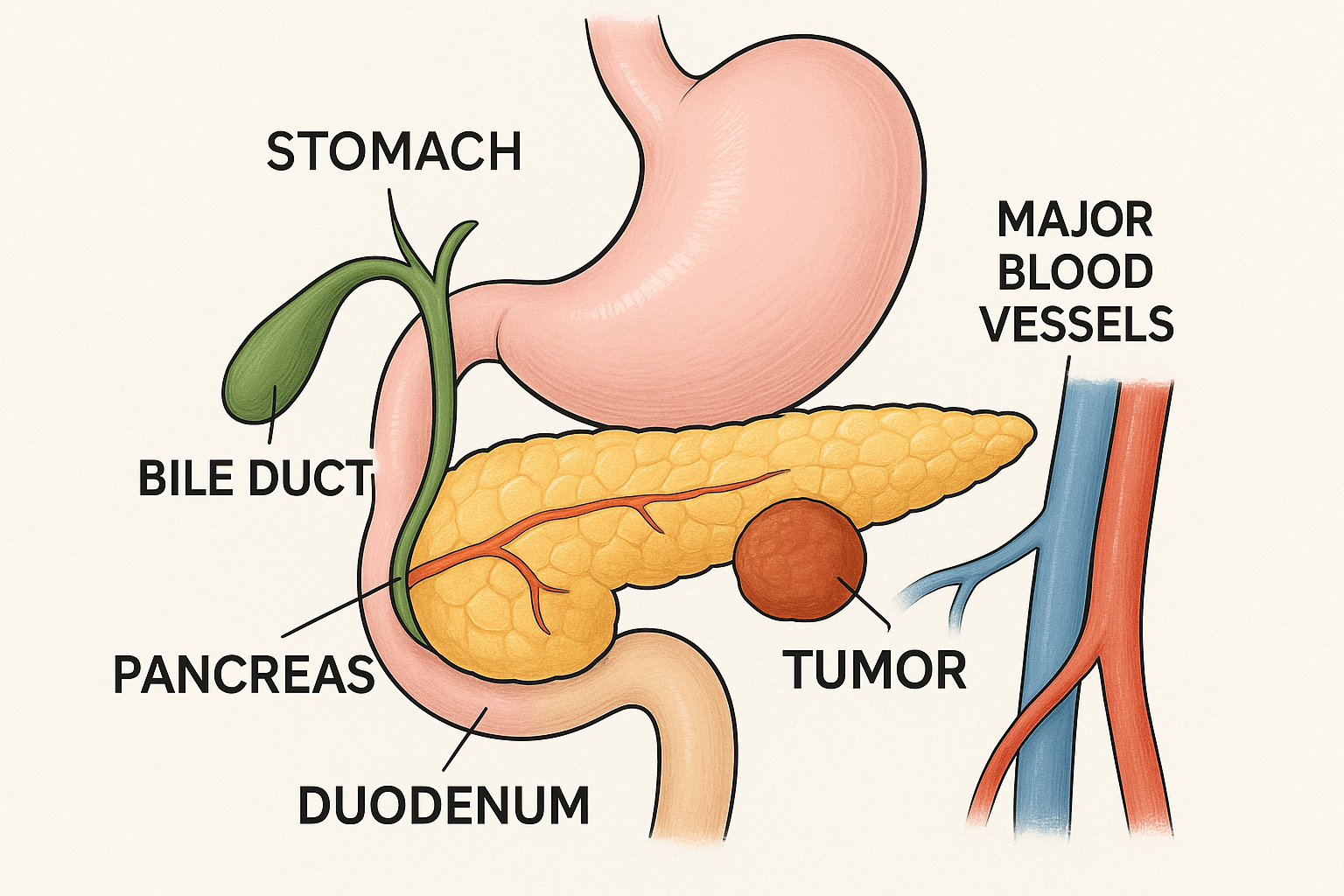
Figure 1: Anatomical illustration showing pancreatic tumor location and surrounding structures
Anatomical Structure
The pancreas is a mixed endocrine and exocrine gland located in the retroperitoneal space, measuring approximately 12-15 cm in length. Understanding its anatomical relationships is essential for comprehending how pancreatic tumors affect surrounding structures and cause characteristic symptoms.
| Anatomical Region | Location | Key Relationships | Clinical Significance |
|---|---|---|---|
| Head | Right side, surrounded by duodenal C-loop | Common bile duct, portal vein, SMA | Most common tumor location (65%) |
| Body | Central portion, behind stomach | Celiac axis, splenic vessels | 20% of tumors, often larger at diagnosis |
| Tail | Left side, near spleen | Splenic vessels, left kidney | 15% of tumors, better surgical outcomes |
Physiological Functions
🥄 Exocrine Function
Produces 1.5-2L of pancreatic juice daily containing enzymes for protein, fat, and carbohydrate digestion
🍯 Endocrine Function
Islets of Langerhans produce insulin, glucagon, and somatostatin for glucose homeostasis
Protease production
Amylase secretion
Nutrient absorption aid
Carbohydrate enzyme release
Regulation of blood glucose
Endocrine hormone synthesis
Alkaline juice neutralization
Steatolysis through lipase
🔬 Pathophysiology
The development of pancreatic tumors involves a complex interplay of genetic mutations, environmental factors, and cellular transformation processes. Understanding these mechanisms helps nurses appreciate the aggressive nature of these malignancies and the rationale behind treatment approaches.
Molecular Pathogenesis
Pancreatic adenocarcinoma typically develops through a progression model involving pancreatic intraepithelial neoplasia (PanIN) lesions that gradually acquire oncogenic mutations. The most commonly affected genes include KRAS, TP53, CDKN2A, and SMAD4, which collectively drive tumor initiation, progression, and metastasis.
🧬 Key Genetic Alterations
- KRAS mutations: Present in 90-95% of cases, driving cellular proliferation
- TP53 mutations: Found in 70-80% of cases, disrupting DNA repair
- CDKN2A inactivation: Occurs in 80-90% of cases, affecting cell cycle control
- SMAD4 loss: Present in 50% of cases, impairing growth inhibition
Tumor Microenvironment
Pancreatic tumors are characterized by an extensive desmoplastic reaction, creating a dense fibrous stroma that constitutes up to 80% of the tumor mass. This unique microenvironment contributes to treatment resistance by:
- Creating physical barriers that impede drug delivery
- Promoting hypoxia and metabolic stress
- Facilitating immune evasion mechanisms
- Supporting tumor cell survival and metastasis
⚠️ Clinical Correlation
The dense stroma explains why pancreatic tumors often appear hypovascular on imaging studies and why traditional chemotherapy has limited efficacy. This understanding helps nurses explain to patients why treatment response may be slower compared to other cancer types.
🏷️ Types of Pancreatic Tumors
Pancreatic tumors encompass a diverse group of malignancies with varying clinical presentations, treatment approaches, and prognoses. Proper classification is essential for appropriate nursing care planning and patient education.
| Tumor Type | Frequency | Origin | Prognosis | Key Features |
|---|---|---|---|---|
| Adenocarcinoma | 85-90% | Ductal epithelium | Poor (5-year: 10%) | Aggressive, early metastasis |
| Neuroendocrine Tumors | 3-5% | Islet cells | Variable (5-year: 60-90%) | May be functional/non-functional |
| Acinar Cell Carcinoma | 1-2% | Acinar cells | Intermediate | Rare, better than adenocarcinoma |
| Cystic Neoplasms | 2-3% | Various | Variable | May be benign or malignant |
Functional Neuroendocrine Tumors
These rare tumors produce hormones that cause specific clinical syndromes, requiring specialized nursing assessment and management strategies:
🍯 Insulinoma
Whipple’s triad: hypoglycemic symptoms, low glucose levels, symptom relief with glucose administration
🌊 Gastrinoma
Zollinger-Ellison syndrome: peptic ulcers, diarrhea, elevated gastrin levels
🎭 Glucagonoma
Necrolytic migratory erythema, diabetes, weight loss, anemia
💧 VIPoma
WDHA syndrome: watery diarrhea, hypokalemia, achlorhydria
⚠️ Risk Factors
Understanding risk factors for pancreatic tumors enables nurses to identify high-risk patients, promote preventive measures, and provide targeted education. Risk factors can be categorized as modifiable and non-modifiable.
Non-Modifiable Risk Factors
| Risk Factor | Relative Risk | Description | Nursing Implications |
|---|---|---|---|
| Age | Increases with age | 90% occur after age 55 | Enhanced screening for elderly patients |
| Male Gender | 1.3x higher | Slightly higher incidence in males | Gender-specific risk assessment |
| Ethnicity | Varies | Higher in African Americans | Cultural competency in care |
| Family History | 2-3x higher | Genetic predisposition | Genetic counseling referral |
Modifiable Risk Factors
🚭 Lifestyle Factors
- Smoking: 2-3 fold increased risk, dose-dependent relationship
- Obesity: BMI >30 increases risk by 20-40%
- Diet: High red meat, low fruit/vegetable intake
- Alcohol: Heavy consumption (>3 drinks/day) increases risk
- Physical inactivity: Sedentary lifestyle contributes to risk
Medical Conditions
- Diabetes mellitus: 2-fold increased risk, may be early symptom
- Chronic pancreatitis: 5-10 fold increased risk
- Hereditary pancreatitis: 50-70 fold increased risk
- Helicobacter pylori infection: Modest increased risk
Smoking tobacco
Male gender
Obesity and diet
Kchronic pancreatitis
Iincreasing age
New-onset diabetes
Genetic predisposition
🩺 Clinical Manifestations
The clinical presentation of pancreatic tumors varies significantly based on tumor location, size, and stage. Early-stage disease is often asymptomatic, contributing to the poor prognosis associated with these malignancies. As nurses, recognizing subtle signs and understanding the progression of symptoms is crucial for early identification and prompt intervention.
Early Signs and Symptoms
Unfortunately, pancreatic tumors rarely produce specific early symptoms, making early detection extremely challenging. However, nurses should be alert to subtle changes that may indicate developing disease:
🍽️ Vague Abdominal Discomfort
Non-specific epigastric pain, often dismissed as dyspepsia or gastritis
⚖️ Unexplained Weight Loss
Gradual, progressive weight loss without obvious cause
🍯 New-Onset Diabetes
Sudden development of diabetes in patients >50 years without family history
😴 Fatigue and Malaise
Non-specific but persistent feeling of tiredness and weakness
Advanced Disease Manifestations
As pancreatic tumors progress, more specific and severe symptoms develop, often leading to initial diagnosis:
| Symptom Category | Specific Signs | Frequency | Nursing Assessment Focus |
|---|---|---|---|
| Pain | Severe epigastric pain radiating to back | 70-80% | Pain intensity, quality, radiation pattern |
| Jaundice | Progressive painless jaundice | 60-70% (head tumors) | Skin/scleral color, urine/stool changes |
| Weight Loss | Significant unintentional weight loss | 85-90% | Baseline weight, nutritional status |
| Digestive Issues | Steatorrhea, nausea, vomiting | 50-60% | Bowel patterns, dietary tolerance |
Tumor Location-Specific Symptoms
🎯 Head of Pancreas Tumors (65% of cases)
- Courvoisier’s sign: Palpable, non-tender gallbladder with jaundice
- Progressive jaundice: Often the presenting symptom
- Clay-colored stools: Due to bile duct obstruction
- Dark urine: Elevated conjugated bilirubin
- Pruritus: Intense itching from bile salt accumulation
🎯 Body/Tail Tumors (35% of cases)
- Severe back pain: Due to celiac plexus involvement
- Left upper quadrant mass: Palpable tumor
- Splenomegaly: From splenic vein compression
- Later presentation: Symptoms appear when tumor is larger
Paraneoplastic Syndromes
Some patients develop symptoms unrelated to direct tumor effects:
- Trousseau’s syndrome: Migratory thrombophlebitis
- Depression: Often precedes other symptoms by months
- Diabetes mellitus: New-onset or worsening glucose control
- Panniculitis: Subcutaneous fat necrosis
🚨 Red Flag Symptoms
Nurses should immediately alert healthcare providers when patients present with:
- Painless progressive jaundice in patients >40 years
- New-onset diabetes with significant weight loss
- Severe epigastric pain radiating to the back
- Unexplained thromboembolism with abdominal symptoms
Painful epigastric/back pain
Abdominal mass (late sign)
Icterus (jaundice)
New-onset diabetes
Fatigue and weakness
Unintentional weight loss
Loss of appetite (anorexia)
🔍 Diagnostic Methods
Diagnosing pancreatic tumors requires a comprehensive approach combining clinical assessment, laboratory studies, and advanced imaging techniques. Early and accurate diagnosis is crucial for determining treatment options and prognosis.
Laboratory Studies
| Test Category | Specific Tests | Expected Findings | Clinical Significance |
|---|---|---|---|
| Tumor Markers | CA 19-9, CEA | CA 19-9 >37 U/mL (elevated in 70-90%) | Monitoring treatment response |
| Liver Function | Bilirubin, ALT, AST, ALP | Elevated in biliary obstruction | Indicates bile duct involvement |
| Pancreatic Function | Lipase, amylase | May be normal or elevated | Assess pancreatic compromise |
| Metabolic Panel | Glucose, albumin, prealbumin | Hyperglycemia, hypoalbuminemia | Nutritional and metabolic status |
Imaging Studies
🖼️ First-Line Imaging
- CT with contrast: Most important initial study, 90-95% sensitivity
- Triphasic technique: Arterial, pancreatic, and portal venous phases
- Dual-phase pancreatic protocol: Optimal visualization of pancreatic parenchyma
Advanced Diagnostic Procedures
When initial imaging is inconclusive or tissue diagnosis is needed:
🔬 ERCP
Endoscopic retrograde cholangiopancreatography for biliary/pancreatic duct evaluation
🎯 EUS
Endoscopic ultrasound with fine-needle aspiration for tissue sampling
🧲 MRCP
Magnetic resonance cholangiopancreatography for detailed ductal imaging
☢️ PET-CT
Positron emission tomography for metastasis detection and staging
Tissue Diagnosis
Definitive diagnosis requires histopathological confirmation:
- EUS-guided FNA: Preferred method for tissue sampling
- CT-guided biopsy: Alternative when EUS unavailable
- Surgical biopsy: Rarely needed, reserved for specific cases
- Cytology: From biliary brushings or pancreatic juice
⚠️ Nursing Considerations for Diagnostic Procedures
- Pre-procedure: NPO status, consent, allergy assessment
- Contrast precautions: Renal function, metformin hold
- Post-procedure monitoring: Vital signs, pain assessment
- Complication surveillance: Bleeding, perforation, pancreatitis
📊 Staging & Prognosis
Accurate staging of pancreatic tumors is essential for treatment planning and prognostic assessment. The TNM classification system and resectability status are the primary determinants of therapeutic approach and expected outcomes.
TNM Staging System
| Stage | T Status | N Status | M Status | 5-Year Survival |
|---|---|---|---|---|
| Stage IA | T1 | N0 | M0 | 20-30% |
| Stage IB | T2 | N0 | M0 | 15-25% |
| Stage IIA | T3 | N0 | M0 | 10-15% |
| Stage IIB | T1-3 | N1 | M0 | 8-12% |
| Stage III | T4 | Any N | M0 | 3-5% |
| Stage IV | Any T | Any N | M1 | 1-3% |
Resectability Classification
More clinically relevant than TNM staging for treatment decisions:
🟢 Resectable (15-20% of patients)
- No distant metastases
- No arterial involvement (celiac, SMA, hepatic artery)
- No venous involvement or minimal involvement suitable for reconstruction
- Adequate performance status for major surgery
🟡 Borderline Resectable (15-20% of patients)
- Limited arterial involvement (<180° circumference)
- Venous involvement requiring reconstruction
- May benefit from neoadjuvant therapy
- Requires multidisciplinary team evaluation
🔴 Unresectable (60-70% of patients)
- Distant metastases (liver, peritoneum, lungs)
- Extensive arterial involvement (>180° circumference)
- Venous occlusion without reconstruction option
- Poor performance status
Prognostic Factors
Multiple factors influence prognosis beyond stage:
| Factor | Favorable | Unfavorable | Impact on Survival |
|---|---|---|---|
| Tumor Size | <2 cm | >4 cm | Smaller tumors have better outcomes |
| Lymph Nodes | N0 (no involvement) | N2 (>3 nodes involved) | Node-negative disease improves survival |
| Margins | R0 (negative) | R1/R2 (positive) | Complete resection essential |
| CA 19-9 | <37 U/mL | >1000 U/mL | Marker correlates with burden |
Size of tumor
Tumor markers (CA 19-9)
Arterial involvement
Grade and differentiation
Extent of lymph node involvement
Surgical margins
🩺 Comprehensive Nursing Assessment
A thorough nursing assessment forms the foundation of effective care for patients with pancreatic tumors. This assessment must be systematic, comprehensive, and ongoing, addressing both physical and psychosocial aspects of the patient’s condition.
Primary Assessment Components
💓 Cardiovascular Status
Blood pressure, heart rate, peripheral pulses, signs of fluid overload or dehydration
🫁 Respiratory Function
Respiratory rate, oxygen saturation, breath sounds, dyspnea assessment
🧠 Neurological Status
Level of consciousness, confusion, depression screening, cognitive function
🍽️ Nutritional Assessment
Weight trends, BMI, albumin levels, dietary intake, swallowing ability
Detailed Physical Assessment
| System | Assessment Focus | Key Findings | Nursing Implications |
|---|---|---|---|
| Integumentary | Skin color, turgor, lesions | Jaundice, pruritus, excoriation | Skin care protocols, comfort measures |
| Gastrointestinal | Abdominal exam, bowel sounds | Mass, distension, steatorrhea | Nutritional support, enzyme therapy |
| Genitourinary | Urine output, color, specific gravity | Dark urine, oliguria | Fluid balance monitoring |
| Musculoskeletal | Muscle mass, strength, mobility | Wasting, weakness, pain | Physical therapy, fall prevention |
Pain Assessment
Pain is a dominant symptom requiring comprehensive evaluation:
🎯 PQRST Pain Assessment for Pancreatic Tumors
- Provocation/Palliation: What makes pain better/worse?
- Quality: Describe the pain (burning, gnawing, stabbing)
- Radiation: Does pain spread to back, shoulder, or other areas?
- Severity: Rate pain on 0-10 scale
- Timing: When does pain occur? Duration? Pattern?
Psychosocial Assessment
The psychological impact of pancreatic tumor diagnosis is profound and requires careful evaluation:
- Emotional response: Anxiety, depression, fear, anger
- Coping mechanisms: Previous strategies, current effectiveness
- Support systems: Family, friends, spiritual resources
- Understanding of diagnosis: Knowledge level, misconceptions
- Decision-making capacity: Ability to participate in care planning
Functional Status Assessment
Evaluate the patient’s ability to perform activities of daily living:
🏃 Performance Status
ECOG or Karnofsky scale assessment for treatment planning
🛁 ADL Independence
Bathing, dressing, eating, toileting capabilities
🏠 IADL Function
Shopping, cooking, medication management, transportation
⚖️ Fall Risk
Mobility, balance, environmental hazards assessment
🚨 Critical Assessment Priorities
Immediate nursing assessment should focus on:
- Airway, breathing, circulation stability
- Pain intensity and management needs
- Nutritional status and risk for malnutrition
- Fluid and electrolyte balance
- Risk for bleeding or infection
- Psychological distress and support needs
Documentation and Communication
Effective documentation ensures continuity of care and supports clinical decision-making:
- Baseline measurements: Establish trends for comparison
- Symptom trajectory: Document changes over time
- Response to interventions: Effectiveness of treatments
- Patient goals: Preferences and priorities
- Family involvement: Support system dynamics
📋 Priority Nursing Diagnoses
Nursing diagnoses for patients with pancreatic tumors must address the complex, multisystem effects of the disease and its treatment. Priority diagnoses focus on life-threatening conditions while also addressing quality of life concerns.
Primary Nursing Diagnoses
| Priority Level | Nursing Diagnosis | Related Factors | Expected Outcomes |
|---|---|---|---|
| High | Acute/Chronic Pain | Tumor compression, nerve involvement | Pain <4/10, improved function |
| High | Imbalanced Nutrition: Less than body requirements | Malabsorption, anorexia, obstruction | Stable weight, adequate intake |
| High | Risk for Deficient Fluid Volume | Poor intake, losses, third spacing | Normal hydration status |
| Moderate | Anxiety/Fear | Life-threatening diagnosis, prognosis | Effective coping strategies |
| Moderate | Activity Intolerance | Fatigue, weakness, pain | Improved endurance, safety |
Detailed Diagnosis Development
🎯 Acute Pain Related to Tumor Growth and Tissue Compression
Assessment Data:
- Patient reports severe epigastric pain 8/10
- Pain radiates to back, worsens with eating
- Grimacing, guarding behaviors observed
- Sleep disturbance due to pain
Expected Outcomes:
- Patient will report pain level <4/10 within 24 hours
- Patient will demonstrate relaxed body posture
- Patient will sleep 6-8 hours without pain interruption
- Patient will participate in ADLs with minimal pain
🍽️ Imbalanced Nutrition: Less Than Body Requirements
Assessment Data:
- 15% weight loss over 3 months
- Decreased appetite, early satiety
- Steatorrhea, malabsorption symptoms
- Low albumin and prealbumin levels
Expected Outcomes:
- Patient will maintain current weight or gain 1-2 lbs/week
- Patient will consume 75% of prescribed diet
- Patient will have improved laboratory nutrition markers
- Patient will report increased energy levels
Secondary Nursing Diagnoses
Additional diagnoses that may apply based on individual patient presentation:
😟 Ineffective Coping
Related to life-threatening diagnosis and uncertain prognosis
🛡️ Risk for Infection
Related to malnutrition, invasive procedures, immunosuppression
🩸 Risk for Bleeding
Related to liver dysfunction, anticoagulation, procedures
🏠 Impaired Home Maintenance
Related to fatigue, weakness, treatment demands
Collaborative Problems
Potential complications requiring interdisciplinary management:
- PC: Biliary obstruction – Monitor for jaundice, liver function changes
- PC: Bowel obstruction – Assess for nausea, vomiting, distension
- PC: Thromboembolism – Monitor for DVT, PE signs
- PC: Diabetes mellitus – Blood glucose monitoring, insulin management
- PC: Electrolyte imbalances – Hyponatremia, hypokalemia risk
Pain (acute/chronic)
Anxiety and fear
Nutrition imbalance
Coping ineffective
Risk for infection
Energy/activity intolerance
Airway clearance (if applicable)
Skin integrity impairment
🎯 Individualized Care Planning
Remember that nursing diagnoses must be individualized based on each patient’s unique presentation, stage of disease, treatment plan, and personal circumstances. Regular reassessment and diagnosis revision are essential as the patient’s condition evolves.
💊 Evidence-Based Nursing Interventions
Nursing interventions for patients with pancreatic tumors must be comprehensive, evidence-based, and tailored to the individual patient’s needs. These interventions span across multiple domains including pain management, nutritional support, psychosocial care, and complication prevention.
Pain Management Interventions
Effective pain control is paramount for patient comfort and quality of life:
| Intervention Category | Specific Actions | Rationale | Evaluation Criteria |
|---|---|---|---|
| Pharmacological | Administer opioids, adjuvants per protocol | Multi-modal approach addresses different pain mechanisms | Pain score <4/10 |
| Positioning | Semi-Fowler’s, knees flexed, side-lying | Reduces pressure on celiac plexus | Patient reports comfort |
| Heat/Cold Therapy | Warm packs to abdomen, ice to back | Gate control theory, muscle relaxation | Decreased muscle tension |
| Complementary | Guided imagery, relaxation, music | Reduces anxiety, enhances coping | Improved sleep, mood |
Nutritional Support Interventions
Addressing malnutrition is critical for treatment tolerance and outcomes:
🍽️ Comprehensive Nutrition Protocol
- Assessment: Daily weight, I&O, dietary intake assessment
- Enzyme replacement: Pancrelipase with meals and snacks
- Diet modification: Small, frequent, high-calorie, low-fat meals
- Supplementation: Fat-soluble vitamins, protein supplements
- Alternative feeding: Enteral or parenteral nutrition if indicated
Symptom-Specific Interventions
🟨 Jaundice Management
- Monitor bilirubin levels
- Provide tepid baths for pruritus
- Apply moisturizers
- Administer antihistamines
🤢 Nausea/Vomiting Control
- Antiemetics before meals
- Small, frequent feedings
- Avoid strong odors
- Cold/room temperature foods
💩 Steatorrhea Management
- Pancreatic enzyme replacement
- Fat-restricted diet
- Medium-chain triglycerides
- Perianal skin care
😴 Fatigue Interventions
- Energy conservation techniques
- Planned rest periods
- Activity scheduling
- Sleep hygiene education
Psychosocial Support Interventions
Addressing the emotional and psychological aspects of pancreatic tumor diagnosis:
- Active listening: Provide time for patient to express fears and concerns
- Information provision: Explain procedures, treatments, and expected outcomes
- Support group referrals: Connect with other patients and families
- Spiritual care: Facilitate chaplain visits or spiritual practices
- Family involvement: Include family in care planning and education
- Professional counseling: Refer to social work, psychology as needed
Complication Prevention
Proactive interventions to prevent common complications:
🛡️ Infection Prevention Bundle
- Hand hygiene compliance
- Aseptic technique for procedures
- Monitor for signs of infection
- Maintain skin integrity
- Ensure adequate nutrition
- Encourage mobility and deep breathing
Patient and Family Education
Comprehensive education empowers patients and families to participate in care:
| Education Topic | Key Points | Teaching Method | Evaluation |
|---|---|---|---|
| Disease Process | Anatomy, tumor effects, prognosis | Visual aids, written materials | Return demonstration, Q&A |
| Medication Management | Dosing, timing, side effects | Pill organizers, schedules | Medication reconciliation |
| Nutrition Guidelines | Enzyme timing, diet modifications | Dietitian consultation | Food diary review |
| Symptom Management | When to call provider, home remedies | Written instructions, role play | Scenario-based questions |
🎯 Intervention Priorities
Always prioritize interventions based on:
- Life-threatening conditions first (bleeding, obstruction)
- Patient-identified priorities and goals
- Evidence-based effectiveness
- Available resources and support systems
- Patient’s functional status and prognosis
Communication and support
Optimal pain management
Maintain nutrition and hydration
Family involvement and education
Organize comprehensive care
Risk reduction strategies
Teaching and learning facilitation
💊 Pharmacological Management
Medication management for patients with pancreatic tumors involves multiple drug categories addressing pain control, nutritional support, symptom management, and cancer treatment. Nurses play a crucial role in administration, monitoring, and patient education regarding these complex medication regimens.
Pain Management Medications
Pain control often requires multimodal therapy with various medication classes:
| Medication Class | Examples | Mechanism | Nursing Considerations |
|---|---|---|---|
| Opioid Analgesics | Morphine, Oxycodone, Fentanyl | Mu-opioid receptor agonists | Monitor respiratory status, constipation management |
| Adjuvant Analgesics | Gabapentin, Pregabalin | Neuropathic pain control | Sedation monitoring, gradual titration |
| Corticosteroids | Dexamethasone, Prednisone | Anti-inflammatory, appetite stimulant | Blood glucose monitoring, infection risk |
| Topical Agents | Lidocaine patches, Capsaicin | Local anesthetic effects | Skin integrity assessment, application technique |
Pancreatic Enzyme Replacement
Essential for managing malabsorption and maintaining nutritional status:
💊 Pancrelipase (Creon, Pancreaze, Zenpep)
Mechanism: Replaces deficient pancreatic enzymes (lipase, protease, amylase)
Dosing: 25,000-50,000 units lipase with meals, 10,000-25,000 units with snacks
Administration:
- Take with first bite of food
- Swallow capsules whole or sprinkle on acidic food
- Do not crush or chew enteric-coated beads
- Adjust dose based on stool consistency and fat content
Monitoring: Stool frequency, consistency, weight trends, nutritional markers
Antiemetic Medications
Nausea and vomiting management using multiple pathways:
🧠 5-HT3 Antagonists
Ondansetron, Granisetron
Block serotonin receptors in CTZ
Monitor for constipation, headache
🎯 NK1 Antagonists
Aprepitant
Block substance P receptors
Check drug interactions, liver function
🌟 Dopamine Antagonists
Metoclopramide, Haloperidol
Block dopamine receptors
Monitor for extrapyramidal effects
💤 Sedating Agents
Lorazepam, Dronabinol
Anticipatory nausea control
Assess sedation, respiratory status
Chemotherapy Agents
Systemic therapy options vary based on performance status and disease stage:
| Regimen | Components | Indication | Key Side Effects |
|---|---|---|---|
| FOLFIRINOX | 5-FU, Leucovorin, Irinotecan, Oxaliplatin | Metastatic disease, good PS | Neutropenia, neuropathy, diarrhea |
| Gemcitabine/Abraxane | Gemcitabine + Paclitaxel | First-line metastatic | Myelosuppression, neuropathy |
| Gemcitabine Monotherapy | Gemcitabine alone | Poor PS, elderly patients | Flu-like symptoms, rash |
Supportive Care Medications
Additional medications to manage symptoms and complications:
- Proton pump inhibitors: Omeprazole, pantoprazole for gastric protection
- Anticoagulants: Enoxaparin for thromboembolism prevention
- Antidiabeetics: Insulin for new-onset diabetes management
- Bowel regimen: Senna, docusate for opioid-induced constipation
- Appetite stimulants: Megestrol acetate, dronabinol
Medication Safety and Monitoring
Critical nursing responsibilities for safe medication management:
🔍 Key Monitoring Parameters
- Opioids: Respiratory rate, sedation level, bowel function
- Chemotherapy: CBC, liver function, renal function
- Enzymes: Stool patterns, weight, nutritional status
- Steroids: Blood glucose, signs of infection, mood changes
⚠️ Drug Interaction Alerts
High-risk interactions to monitor:
- Warfarin + chemotherapy (bleeding risk)
- Metformin + contrast agents (nephrotoxicity)
- CYP3A4 inhibitors + opioids (enhanced sedation)
- Proton pump inhibitors + enzyme replacement (reduced effectiveness)
Patient Education for Medications
Comprehensive education ensures safe and effective medication use:
📋 Administration Guidelines
Timing, food interactions, proper technique for each medication
⚠️ Side Effect Recognition
When to call provider, emergency symptoms, management strategies
💊 Storage and Handling
Temperature requirements, expiration dates, safe disposal
📞 Communication
Medication lists, allergy alerts, provider contact information
🏥 Surgical Care and Management
Surgical intervention offers the only potential cure for pancreatic tumors, though only 15-20% of patients are candidates for resection at diagnosis. Nursing care for surgical patients requires expertise in complex perioperative management and recognition of potential complications.
Types of Pancreatic Surgery
| Procedure | Indication | Structures Removed | Major Complications |
|---|---|---|---|
| Whipple (PPPD) | Head of pancreas tumors | Pancreatic head, duodenum, GB, part of stomach | Leak, bleeding, DGE |
| Distal Pancreatectomy | Body/tail tumors | Pancreatic body/tail, often spleen | Leak, diabetes, bleeding |
| Total Pancreatectomy | Multifocal disease | Entire pancreas, spleen, duodenum | Brittle diabetes, malabsorption |
| Enucleation | Small benign lesions | Tumor only | Leak, minimal morbidity |
Preoperative Nursing Care
Comprehensive preparation for major pancreatic surgery:
🔍 Preoperative Assessment Priorities
- Nutritional status: Albumin, prealbumin, weight trends
- Functional capacity: Performance status, cardiac evaluation
- Biliary drainage: ERCP/PTC stent function if present
- Diabetes management: Glucose control optimization
- Smoking cessation: Minimum 4 weeks before surgery
Intraoperative Considerations
Key nursing awareness of intraoperative factors affecting postoperative care:
- Surgery duration: 4-8 hours for major resections
- Blood loss: Potential for significant transfusion requirements
- Anatomical reconstruction: Multiple anastomoses created
- Drain placement: Typically 2-3 drains for monitoring
- Vascular involvement: May require vessel reconstruction
Postoperative Nursing Care
Intensive monitoring and specialized care in the immediate postoperative period:
💓 Hemodynamic Monitoring
- Vital signs every 15 minutes initially
- CVP/arterial line monitoring
- Urine output >0.5 mL/kg/hr
- Signs of bleeding or shock
🩸 Drain Management
- Monitor output quantity and quality
- Assess for pancreatic leak
- Amylase levels in drain fluid
- Maintain patency and position
🤢 GI Function
- NGT decompression initially
- Bowel sound assessment
- Progressive diet advancement
- Monitor for delayed gastric emptying
🍯 Glucose Control
- Frequent blood glucose monitoring
- Insulin protocols per guidelines
- Signs of hypo/hyperglycemia
- Endocrine consultation
Major Postoperative Complications
Early recognition and management of life-threatening complications:
| Complication | Incidence | Signs/Symptoms | Nursing Actions |
|---|---|---|---|
| Pancreatic Leak | 10-20% | High-amylase drain output, fever, pain | Monitor drains, fluid balance, prepare for intervention |
| Delayed Gastric Emptying | 20-40% | Persistent NGT output, nausea, inability to eat | Prokinetic agents, nutritional support, patience |
| Postoperative Bleeding | 5-10% | Hemodynamic instability, bloody drains, anemia | Transfusion preparation, surgical consultation |
| Intra-abdominal Abscess | 5-15% | Fever, leukocytosis, abdominal pain | Blood cultures, imaging, antibiotic therapy |
Recovery and Rehabilitation
Supporting patients through the complex recovery process:
📈 Recovery Milestones
- POD 1-3: ICU monitoring, hemodynamic stability
- POD 4-7: Drain removal, diet advancement
- POD 7-10: Discharge planning, medication education
- 2-4 weeks: Outpatient follow-up, pathology results
- 4-6 weeks: Adjuvant therapy consideration
Discharge Planning
Comprehensive preparation for safe transition home:
- Medication reconciliation: Pain management, enzymes, diabetes medications
- Diet education: Enzyme timing, small frequent meals, restrictions
- Activity guidelines: Lifting restrictions, gradual progression
- Incision care: Signs of infection, when to call provider
- Follow-up appointments: Surgeon, oncologist, primary care
- Emergency contacts: 24-hour nursing line, emergency department
🚨 Emergency Warning Signs
Educate patients to seek immediate medical attention for:
- Severe abdominal pain different from surgical pain
- Fever >101°F (38.3°C) or chills
- Nausea/vomiting preventing oral intake
- Signs of incision infection or dehiscence
- Severe hypoglycemia or hyperglycemia
⚠️ Complications Management
Patients with pancreatic tumors are at high risk for multiple complications related to both the disease process and its treatment. Early recognition and prompt intervention are crucial for optimizing outcomes and maintaining quality of life.
Disease-Related Complications
🟨 Biliary Obstruction
Signs: Progressive jaundice, dark urine, pale stools
Management: ERCP with stenting, surgical bypass
Nursing: Monitor bilirubin, skin care for pruritus
🤢 Gastric Outlet Obstruction
Signs: Persistent vomiting, inability to eat
Management: NGT decompression, enteral stenting
Nursing: I&O monitoring, nutritional support
🩸 Thromboembolism
Signs: Leg swelling, chest pain, dyspnea
Management: Anticoagulation, supportive care
Nursing: DVT prevention, bleeding precautions
🍯 New-Onset Diabetes
Signs: Hyperglycemia, polyuria, polydipsia
Management: Insulin therapy, dietary modification
Nursing: Blood glucose monitoring, education
Treatment-Related Complications
| Complication | Risk Factors | Prevention Strategies | Management |
|---|---|---|---|
| Chemotherapy-Induced Neuropathy | Oxaliplatin, paclitaxel use | Dose modification, neuroprotectants | Gabapentin, duloxetine, dose reduction |
| Neutropenia | FOLFIRINOX, poor nutritional status | Growth factors, dose adjustments | G-CSF, infection precautions |
| Mucositis | 5-FU, poor oral hygiene | Oral care protocols, prophylaxis | Pain management, nutritional support |
| Radiation Dermatitis | External beam radiation therapy | Skin care education, gentle products | Topical steroids, wound care |
Nutritional Complications
Malnutrition is both a consequence and risk factor for poor outcomes:
🍽️ Malabsorption Syndrome Management
- Fat-soluble vitamin deficiency: A, D, E, K supplementation
- Protein-energy malnutrition: High-calorie supplements
- Micronutrient deficiencies: B12, folate, iron monitoring
- Bone health: Calcium, vitamin D, bone density screening
Psychosocial Complications
The psychological burden of pancreatic tumor diagnosis is substantial:
- Depression: Screen with PHQ-9, provide counseling resources
- Anxiety: Assess coping mechanisms, consider anxiolytics
- Anticipatory grief: Support patient and family processing
- Social isolation: Encourage support group participation
- Financial stress: Social work referral, resource identification
Emergency Complications
Life-threatening situations requiring immediate intervention:
🚨 Oncological Emergencies
- Superior vena cava syndrome: Facial swelling, dyspnea
- Hypercalcemia: Confusion, constipation, polyuria
- Tumor lysis syndrome: Hyperkalemia, hyperphosphatemia
- Spinal cord compression: Back pain, neurological deficits
- Bowel perforation: Severe abdominal pain, peritonitis
Complication Prevention Strategies
Proactive measures to reduce complication risk:
🛡️ Infection Prevention
- Hand hygiene compliance
- Neutropenia precautions
- Vaccination status review
- Environmental modifications
⚖️ Fall Prevention
- Mobility assessment
- Environmental safety
- Medication review
- Assistive devices
🫀 Thrombosis Prevention
- Early mobilization
- Compression devices
- Anticoagulation protocols
- Risk stratification
🧠 Cognitive Support
- Mental status monitoring
- Medication reconciliation
- Sleep hygiene
- Family involvement
Monitoring and Assessment
Systematic monitoring protocols for early complication detection:
| Assessment Area | Frequency | Key Parameters | Action Thresholds |
|---|---|---|---|
| Vital Signs | Every 4-8 hours | Temperature, BP, HR, RR, O2 sat | Fever >100.4°F, hypotension |
| Laboratory Values | Per protocol | CBC, CMP, LFTs, coagulation | ANC <1000, bilirubin >3.0 |
| Nutritional Status | Weekly | Weight, intake, albumin | 5% weight loss, albumin <3.0 |
| Functional Status | Daily | ADL performance, mobility | Significant decline from baseline |
Proactive monitoring
Risk factor identification
Early intervention
Vital sign surveillance
Education and awareness
Nutrition optimization
Team collaboration
📚 Patient and Family Education
Comprehensive education empowers patients and families to actively participate in care, recognize complications early, and make informed decisions about treatment options. Education must be ongoing, culturally sensitive, and adapted to individual learning needs and health literacy levels.
Core Educational Topics
| Topic Area | Key Concepts | Teaching Methods | Evaluation Strategies |
|---|---|---|---|
| Disease Understanding | Anatomy, tumor effects, prognosis | Visual aids, models, written materials | Teach-back method, Q&A sessions |
| Treatment Options | Surgery, chemotherapy, radiation, palliative care | Decision aids, pros/cons lists | Treatment preference discussions |
| Symptom Management | Pain control, nutrition, side effects | Demonstration, practice sessions | Symptom diaries, return demonstration |
| Medication Management | Dosing, timing, side effects, interactions | Pill organizers, schedules, apps | Medication reconciliation |
Nutritional Education
Detailed guidance on managing nutritional challenges:
🍽️ Pancreatic Enzyme Replacement Education
- Timing: Take with first bite of food, not before or after
- Dosing: Adjust based on fat content of meal
- Administration: Swallow whole or sprinkle on acidic food
- Storage: Room temperature, protect from moisture
- Monitoring: Stool consistency, weight trends, symptoms
🥗 Dietary Modifications
- Small, frequent meals (6-8 per day)
- Moderate fat restriction
- High-calorie, high-protein foods
- Avoid alcohol and simple sugars
💊 Vitamin Supplementation
- Fat-soluble vitamins (A, D, E, K)
- B-complex vitamins
- Calcium and magnesium
- Iron if deficient
⚖️ Weight Monitoring
- Daily weights at same time
- Food intake diaries
- Symptom tracking
- When to call provider
🍯 Diabetes Management
- Blood glucose monitoring
- Insulin administration
- Hypoglycemia recognition
- Carbohydrate counting
Pain Management Education
Empowering patients to effectively manage pain:
💊 Pain Medication Guidelines
- Around-the-clock dosing: Don’t wait for pain to become severe
- Breakthrough pain: Use short-acting medications as prescribed
- Side effect management: Bowel regimen, anti-nausea medications
- Safety precautions: No driving, avoid alcohol, fall prevention
- Tolerance concerns: Escalating doses may be necessary
When to Seek Medical Attention
Clear guidelines for emergency situations and routine follow-up:
🚨 Emergency Warning Signs
Call 911 or go to emergency department immediately for:
- Difficulty breathing or chest pain
- Severe abdominal pain different from usual
- Signs of severe dehydration
- Confusion or altered mental status
- Severe bleeding or black tarry stools
📞 Call Healthcare Provider for:
- Fever >100.4°F (38°C)
- Persistent nausea/vomiting >24 hours
- Yellowing of skin or eyes
- Unexplained weight loss >5 pounds/week
- New or worsening pain not controlled by medications
- Signs of infection (redness, swelling, drainage)
Family and Caregiver Education
Supporting those who provide care and emotional support:
- Caregiver stress management: Self-care importance, respite resources
- Communication strategies: How to talk about illness, prognosis
- Practical skills: Medication administration, symptom assessment
- End-of-life planning: Advanced directives, goals of care discussions
- Support resources: Support groups, counseling, financial assistance
Educational Materials and Resources
Variety of formats to accommodate different learning preferences:
📖 Written Materials
- Patient handbooks
- Medication guides
- Symptom diaries
- Emergency contact cards
🎥 Multimedia Resources
- Educational videos
- Interactive websites
- Mobile applications
- Virtual reality tools
👥 Support Programs
- Patient support groups
- Peer mentorship programs
- Family education classes
- Online communities
🏥 Professional Resources
- Dietitian consultations
- Social work services
- Chaplain support
- Case management
Cultural Considerations
Adapting education to diverse cultural backgrounds and beliefs:
- Language barriers: Professional interpreters, translated materials
- Health beliefs: Integration with traditional practices when safe
- Family dynamics: Respect for decision-making patterns
- Religious considerations: Spiritual care integration
- Dietary restrictions: Cultural food preferences and restrictions
Explain in simple terms
Demonstrate procedures
Use multiple teaching methods
Confirm understanding
Address individual needs
Teach-back verification
Evaluate and reinforce
🌍 Global Best Practices and Innovations
Examining international approaches to pancreatic tumor care reveals innovative strategies and best practices that can enhance nursing care delivery. These global perspectives offer valuable insights into comprehensive care models and emerging technologies.
European Integrated Care Models
Several European countries have developed comprehensive pancreatic cancer care pathways:
🇳🇱 Netherlands – Pancreatic Cancer Network
- Centralized expertise: High-volume centers for complex cases
- Multidisciplinary teams: Mandatory tumor board review
- Quality metrics: Standardized outcome reporting
- Patient navigation: Dedicated nurse coordinators
- Research integration: Clinical trial enrollment optimization
🇬🇧 United Kingdom – Enhanced Recovery Protocols
- Prehabilitation programs: Preoperative fitness optimization
- Standardized perioperative care: Evidence-based protocols
- Early mobilization: Reduced length of stay
- Nutritional optimization: Specialized dietitian involvement
- Patient-reported outcomes: Quality of life monitoring
Asian Innovations in Technology
Asian countries are leading in technological integration for pancreatic cancer care:
🇯🇵 Japan – AI Diagnostics
Advanced AI algorithms for early detection in high-risk populations, endoscopic image analysis
🇰🇷 South Korea – Telemedicine
Remote monitoring systems, virtual consultations, mobile health applications
🇸🇬 Singapore – Precision Medicine
Genomic profiling, personalized treatment protocols, biomarker-driven therapy
🇨🇳 China – Traditional Integration
Combining traditional Chinese medicine with conventional treatment, acupuncture for symptom management
North American Quality Initiatives
Leading quality improvement programs from North America:
| Initiative | Focus Area | Key Components | Nursing Implications |
|---|---|---|---|
| NCCN Guidelines | Standardized care pathways | Evidence-based protocols, regular updates | Adherence to best practices |
| CoC Accreditation | Cancer center quality | Multidisciplinary care, data collection | Quality metrics tracking |
| ASCO Quality Program | Oncology practice improvement | Quality measures, peer review | Professional development |
Innovative Nursing Roles
Emerging specialized nursing roles in pancreatic cancer care globally:
👩⚕️ Advanced Practice Pancreatic Nurse Specialists
- Australia: Nurse practitioners managing follow-up care
- Canada: Clinical nurse specialists in symptom management
- Germany: Oncology nurses leading patient education programs
- Sweden: Research nurses coordinating clinical trials
Palliative Care Integration
International models for early palliative care integration:
- Switzerland: Automatic palliative care referral at diagnosis
- Norway: Community-based palliative care teams
- Belgium: Integrated palliative care pathways
- New Zealand: Family-centered palliative care models
Research and Innovation Centers
Leading global centers advancing pancreatic cancer research:
🇺🇸 Johns Hopkins
Sol Goldman Pancreatic Cancer Research Center – genetic research, early detection
🇩🇪 Heidelberg University
NCT Heidelberg – precision oncology, immunotherapy trials
🇫🇷 Institut Curie
Molecular profiling, targeted therapy development
🇦🇺 Garvan Institute
Organoid research, drug screening platforms
Global Nursing Education Programs
International initiatives for specialized pancreatic cancer nursing education:
- European Oncology Nursing Society: Specialized pancreatic cancer certification
- International Association of Healthcare: Global competency standards
- World Health Organization: Palliative care training modules
- Pancreatic Cancer Action Network: Nursing education webinars
Telemedicine and Digital Health
Global adoption of digital health solutions:
📱 Digital Health Innovations
- Symptom tracking apps: Real-time monitoring and alerts
- Virtual reality therapy: Pain and anxiety management
- AI-powered chatbots: 24/7 patient support and triage
- Wearable devices: Continuous health monitoring
- Electronic patient-reported outcomes: Quality of life tracking
Global Research Collaborations
International partnerships advancing pancreatic cancer research:
- Pancreatic Cancer Collective: Global data sharing initiative
- International Cancer Genome Consortium: Genomic data collaboration
- Global Alliance for Genomics and Health: Data standardization
- World Pancreatic Cancer Coalition: Patient advocacy and research
🎯 Implementing Global Best Practices
Nurses can advocate for implementation of proven international practices:
- Multidisciplinary team approaches
- Standardized care pathways
- Enhanced recovery protocols
- Early palliative care integration
- Technology-enhanced patient monitoring
- Comprehensive patient navigation programs
Future Directions
Emerging trends in global pancreatic cancer care:
- Liquid biopsies: Blood-based early detection methods
- Immunotherapy combinations: Novel treatment approaches
- Artificial pancreas systems: For post-pancreatectomy patients
- Robotic surgery: Minimally invasive techniques
- Personalized nutrition: Genomics-based dietary recommendations
Guideline-based care
Leadership in innovation
Outcome measurement
Best practice sharing
Adaptation to local context
Lifelong learning commitment
