Rh Incompatibility in Pregnancy
Comprehensive Nursing Notes
Table of Contents
Introduction to Rh Incompatibility
Rh incompatibility is a condition that occurs when a pregnant woman has Rh-negative blood and her fetus has Rh-positive blood. This blood type incompatibility can lead to serious complications if maternal antibodies cross the placenta and attack the fetus’s red blood cells.
Memory Aid: “INCOMPATIBLE”
Immune response occurs when
Negative Rh mom carries
Child with positive Rh type
Only after sensitization
Maternal antibodies cross placenta
Problem affects subsequent pregnancies
Antibodies attack fetal RBCs
Treatment needed to prevent
Immunization with RhoGAM
Blood testing essential
Life-threatening if severe
Erythrocytes (RBCs) are destroyed
Understanding Rh incompatibility is crucial for nursing care of pregnant women, as early detection and prevention are key to avoiding serious consequences for the fetus and newborn.
Pathophysiology of Rh Incompatibility
The Rh Factor
The Rhesus (Rh) factor is a protein found on the surface of red blood cells. Individuals with this protein are classified as Rh-positive, while those without it are Rh-negative. The Rh factor is inherited genetically.
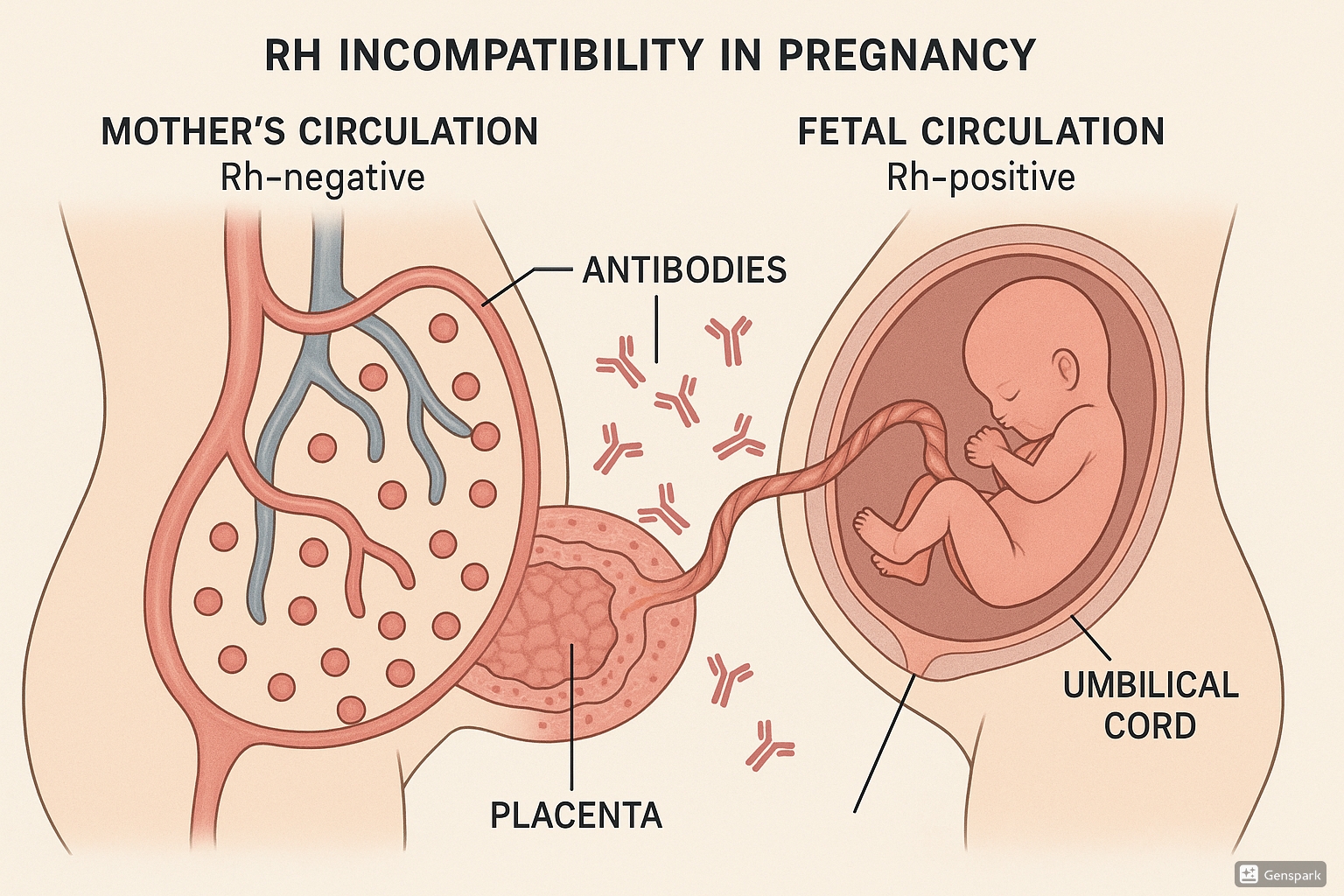
Mechanism of Sensitization
Rh incompatibility occurs when an Rh-negative mother carries an Rh-positive fetus. Under normal circumstances, maternal and fetal circulations are separate. However, small amounts of fetal blood may enter the maternal circulation during:
- Childbirth or cesarean delivery
- Miscarriage or abortion
- Ectopic pregnancy
- Invasive prenatal testing (amniocentesis, CVS)
- Abdominal trauma
- External cephalic version (turning the baby from breech position)
- Antepartum hemorrhage
Immune Response
When fetal Rh-positive red blood cells enter the Rh-negative mother’s circulation, her immune system recognizes the Rh antigen as foreign and produces anti-Rh (anti-D) antibodies. This process is called alloimmunization or sensitization.
Memory Aid: “SENSITIZE”
Small amounts of fetal blood enter maternal circulation
Exposure to Rh-positive cells
Natural immune response activated
Splenic processing of foreign antigens
Immune system creates antibodies
Time needed for antibody production (days to weeks)
IgM antibodies form first, then IgG
Zero effect on first pregnancy usually
Endangers future Rh-positive pregnancies
First vs. Subsequent Pregnancies
In most cases, the first Rh-positive pregnancy in an Rh-negative mother does not pose significant risk to the fetus because:
- Initial sensitization typically occurs during delivery
- The primary immune response is slow, producing mainly IgM antibodies that cannot cross the placenta
- By the time IgG antibodies form, the pregnancy is over
In subsequent Rh-positive pregnancies, the risks increase significantly:
- The mother’s immune system mounts a rapid secondary response
- IgG anti-D antibodies cross the placenta and attach to fetal Rh-positive red blood cells
- This leads to hemolysis (destruction) of fetal RBCs
- The severity increases with each subsequent Rh-positive pregnancy
Clinical Point
A first pregnancy can be affected if sensitization occurs earlier in that pregnancy due to events like amniocentesis, miscarriage, or abdominal trauma. Additionally, a woman may have been sensitized from a previous transfusion with Rh-positive blood.
Clinical Presentation of Rh Incompatibility
Maternal Presentation
The mother typically shows no symptoms related to Rh incompatibility. The condition primarily affects the fetus and newborn.
Fetal Presentation
The severity of fetal presentation varies depending on the degree of hemolysis:
| Severity | Fetal Findings | Pathophysiology |
|---|---|---|
| Mild |
|
Limited hemolysis of fetal RBCs |
| Moderate |
|
Significant hemolysis causing moderate anemia; compensatory mechanisms active |
| Severe |
|
Extensive hemolysis leading to profound anemia; heart failure develops as compensatory mechanisms fail; fluid accumulates in body cavities (hydrops fetalis) |
Neonatal Presentation (Hemolytic Disease of the Newborn)
Newborns affected by Rh incompatibility may present with:
- Anemia: Pallor, tachycardia, poor feeding
- Jaundice: Due to increased bilirubin from RBC breakdown, typically presents within 24 hours of birth (earlier than physiologic jaundice)
- Hepatosplenomegaly: Enlarged liver and spleen due to increased production of RBCs and filtering of damaged cells
- Hyperbilirubinemia: Elevated bilirubin levels that may lead to kernicterus (bilirubin encephalopathy) if untreated
- Hydrops fetalis: Severe edema, ascites, pleural effusions (in severe cases)
- Petechiae: Due to thrombocytopenia
Memory Aid: “JAUNDICE”
Jaundice appears early (within 24 hours)
Anemia from hemolysis
Underdeveloped liver function worsens hyperbilirubinemia
Neurological damage risk (kernicterus)
Direct Coombs test positive
Increased heart rate compensating for anemia
Compensatory erythropoiesis (hepatosplenomegaly)
Edema in severe cases (hydrops)
Long-term Complications
Untreated or severe Rh incompatibility can lead to:
- Kernicterus: Permanent neurological damage from high bilirubin levels
- Cerebral palsy
- Hearing loss
- Developmental delays
- Death (in severe untreated cases)
Diagnosis of Rh Incompatibility
Prenatal Screening
Early identification of at-risk pregnancies is critical for preventing complications of Rh incompatibility.
| Test | Timing | Purpose |
|---|---|---|
| ABO and Rh blood typing | First prenatal visit | Identify Rh-negative mothers |
| Antibody screening | First prenatal visit | Detect existing anti-D antibodies (indicating prior sensitization) |
| Repeat antibody screening | 28 weeks if previously negative | Check for antibody development during pregnancy |
| Paternal blood typing | As needed | Determine risk (if father is Rh-negative, fetus cannot be Rh-positive) |
| Non-invasive fetal Rh genotyping from maternal blood | After 10 weeks gestation | Determine fetal Rh status without invasive testing |
Monitoring Sensitized Pregnancies
If a mother has already developed anti-D antibodies, additional monitoring is required:
- Antibody titers: Measured every 2-4 weeks to assess risk
- Critical titer: Usually 1:16 or 1:32 (varies by laboratory)
- Titers above critical level suggest increased risk of fetal anemia
- Middle Cerebral Artery Peak Systolic Velocity (MCA-PSV): Non-invasive ultrasound measurement used to detect fetal anemia
- Increased velocity indicates anemia (fetus compensating with hyperdynamic circulation)
- Values >1.5 multiples of median (MoM) suggest moderate to severe anemia
- Serial ultrasounds: To detect signs of hydrops fetalis
- Ascites
- Pleural/pericardial effusions
- Skin edema
- Placental thickening
- Polyhydramnios
- Amniocentesis: Used historically to measure amniotic fluid bilirubin levels (ΔOD450), but now largely replaced by MCA-PSV measurements
- Cordocentesis: Direct sampling of fetal blood to measure hemoglobin levels (invasive, used when MCA-PSV results are inconclusive)
Neonatal Diagnosis
After birth, the following tests are used to diagnose hemolytic disease of the newborn:
- Direct Coombs test (DAT): Detects antibodies bound to the surface of the newborn’s RBCs
- Blood type and Rh factor: Confirms the newborn is Rh-positive
- Complete blood count (CBC): Evaluates anemia (hemoglobin/hematocrit)
- Reticulocyte count: Usually elevated due to increased RBC production
- Bilirubin levels: Total and direct (unconjugated) bilirubin to assess severity of hyperbilirubinemia
- Peripheral blood smear: May show nucleated RBCs, spherocytes, and other abnormal RBC morphology
Memory Aid: “DIAGNOSE”
Determine blood type and Rh factor
Identify antibodies through screening
Assess antibody titers if sensitized
Gauge fetal anemia via MCA-PSV
Note signs of hydrops on ultrasound
Observe newborn for jaundice, anemia
Sample blood for Direct Coombs test
Evaluate bilirubin and hemoglobin levels
Management of Rh Incompatibility
Prenatal Management of Sensitized Pregnancies
Once a pregnant woman is sensitized (has developed anti-D antibodies), management focuses on monitoring and treating the fetus:
- Regular monitoring of antibody titers and fetal wellbeing
- Intrauterine transfusion (IUT): The primary treatment for severe fetal anemia
- Performed when MCA-PSV indicates significant anemia
- Typically administered into the umbilical vein under ultrasound guidance
- Provides Rh-negative RBCs to the fetus
- May need to be repeated every 2-4 weeks until delivery
- Timing of delivery:
- Mild cases: May continue to term (37-38 weeks)
- Severe cases requiring IUT: Usually delivered by 35-37 weeks after lung maturity is confirmed
- Critical cases: Early delivery may be necessary, balancing risks of prematurity against risks of continued intrauterine exposure to antibodies
Clinical Point
Intrauterine transfusions have dramatically improved outcomes for severe Rh incompatibility, with survival rates >90% when performed at experienced centers.
Neonatal Management
Management of the affected newborn depends on the severity of hemolytic disease:
| Intervention | Indication | Description |
|---|---|---|
| Phototherapy | Hyperbilirubinemia |
|
| Exchange transfusion |
|
|
| Simple transfusion | Moderate anemia without severe hyperbilirubinemia |
|
| Intravenous immunoglobulin (IVIG) | Severe hemolytic disease |
|
| Supportive care | All affected infants |
|
Nursing Considerations in Management
Nursing care is critical in both prenatal and neonatal management:
- Prenatal care:
- Educating the mother about the condition and monitoring process
- Providing emotional support
- Assisting with fetal monitoring
- Preparing for intrauterine transfusions
- Neonatal care:
- Close monitoring of vital signs
- Frequent assessment for signs of anemia and jaundice
- Proper positioning during phototherapy
- Eye protection during phototherapy
- Temperature regulation
- Adequate hydration
- Monitoring intake and output
- Observing for complications
- Family support:
- Education about the condition and treatment plan
- Promoting parent-infant bonding despite medical interventions
- Addressing concerns about long-term outcomes
Memory Aid: “TREATMENT”
Track antibody titers in sensitized mothers
Regularly monitor MCA-PSV for fetal anemia
Exchange transfusion for severe neonatal disease
Assess bilirubin levels frequently
Timely phototherapy for hyperbilirubinemia
Monitor for complications of treatment
Educate parents about the condition
Nurse in positions that maximize skin exposure during phototherapy
Track hemoglobin and hematocrit levels
Prevention of Rh Incompatibility
Prevention is the cornerstone of managing Rh incompatibility. Since the introduction of RhIg (Rh immune globulin) in the late 1960s, the incidence of Rh sensitization has decreased dramatically.
RhIg (RhoGAM) Administration
RhIg is a blood product containing IgG anti-D antibodies that works by binding to any fetal Rh-positive cells in the maternal circulation, preventing them from triggering the mother’s immune system to produce antibodies.
Important
RhIg is only effective for prevention of sensitization. It cannot treat a mother who has already developed anti-D antibodies.
Indications and Timing for RhIg
RhIg should be administered to Rh-negative, non-sensitized women in the following situations:
| Timing/Event | Dose | Rationale |
|---|---|---|
| Routine antenatal prophylaxis at 28 weeks gestation | 300 μg (standard dose) | Provides protection against small, clinically undetectable fetomaternal hemorrhages during the third trimester |
| Within 72 hours after delivery of Rh-positive infant | 300 μg (standard dose) | Prevents sensitization from fetomaternal hemorrhage during delivery |
| After miscarriage, abortion, or ectopic pregnancy | 50 μg if <12 weeks 300 μg if ≥12 weeks |
Prevents sensitization from potential fetomaternal hemorrhage during pregnancy loss |
| After invasive prenatal procedures (amniocentesis, CVS) | 300 μg | Procedures may cause fetomaternal hemorrhage |
| After abdominal trauma, antepartum hemorrhage | 300 μg | These events may cause fetomaternal hemorrhage |
| After external cephalic version | 300 μg | Procedure may cause fetomaternal hemorrhage |
Nursing Considerations for RhIg Administration
- Verification:
- Confirm mother is Rh-negative
- Confirm absence of anti-D antibodies before administration
- After delivery, verify infant is Rh-positive before administering RhIg
- Administration:
- Administer intramuscularly in the deltoid or ventrogluteal site
- Never administer intravenously
- Observe for 20 minutes after administration for allergic reactions
- Documentation:
- Record lot number, dose, site, time, and patient response
- Document patient education provided
- Patient education:
- Explain purpose of RhIg
- Inform about potential side effects (soreness at injection site, mild fever)
- Emphasize importance of receiving RhIg for all indicated events in current and future pregnancies
Monitoring After Large Fetomaternal Hemorrhage
After delivery, a Kleihauer-Betke test or flow cytometry may be performed to quantify the volume of fetomaternal hemorrhage. If the hemorrhage is large, additional doses of RhIg may be required (one standard dose covers approximately 30 mL of fetal blood).
Memory Aid: “RHOGAM”
Rh-negative mothers need it
Hemorrhage (fetomaternal) prompts administration
On time administration is crucial (within 72 hours)
Given at 28 weeks routinely
After delivery if baby is Rh-positive
Must be administered IM only
Best Practices & Nursing Updates for Rh Incompatibility
Current Best Practices
1. Non-invasive Fetal RhD Genotyping
Cell-free DNA testing from maternal blood can now determine fetal Rh status without invasive procedures. This allows targeted RhIg administration only to Rh-negative women carrying Rh-positive fetuses, reducing unnecessary exposure to blood products.
2. Middle Cerebral Artery Peak Systolic Velocity (MCA-PSV) Monitoring
MCA-PSV has largely replaced amniocentesis for monitoring fetal anemia in sensitized pregnancies. This non-invasive ultrasound technique measures blood flow velocity in the fetal brain, which increases in anemic conditions due to decreased blood viscosity.
3. Intrauterine Transfusion Techniques
Advanced techniques for intrauterine transfusion, including intravascular transfusion via the umbilical cord under ultrasound guidance, have significantly improved outcomes for fetuses with severe anemia due to Rh incompatibility.
Recent Updates in Nursing Care
Several important updates have emerged in the nursing care of patients with Rh incompatibility:
- Universal screening protocols: All pregnant women should receive ABO/Rh screening at first prenatal visit, with antibody screening for Rh-negative women.
- Electronic medical record alerts: Implementation of EMR alerts to ensure timely administration of RhIg for eligible patients.
- Extended window for RhIg effectiveness: While administration within 72 hours is optimal, RhIg may provide some protection when given up to 10-14 days after potential sensitization events.
- Reduced-dose protocols: Some centers are adopting reduced-dose RhIg protocols for early pregnancy events (<12 weeks) to conserve resources while maintaining efficacy.
Nursing Role in Prevention
Nurses play a crucial role in preventing Rh incompatibility complications:
- Education: Educate Rh-negative women about the importance of RhIg prophylaxis and when to seek care for events that may cause fetomaternal hemorrhage.
- Documentation: Maintain accurate records of blood type, antibody screening results, and RhIg administration.
- Communication: Ensure proper handoffs between care settings to prevent missed opportunities for RhIg administration.
- Advocacy: Advocate for timely administration of RhIg when indicated.
- Risk management: Identify and flag Rh-negative patients for special attention during pregnancy and delivery.
Clinical Point
Global inequities in RhIg access persist. According to recent studies, nearly 50% of Rh-negative women worldwide lack access to appropriate immunoprophylaxis, leading to preventable cases of hemolytic disease of the fetus and newborn.
References
- Pegoraro, V., Urbinati, D., Visser, G.H.A., et al. (2020). Hemolytic disease of the fetus and newborn due to Rh(D) incompatibility: A preventable disease that still produces significant morbidity and mortality in children. PLoS ONE, 15(7), e0235807. https://doi.org/10.1371/journal.pone.0235807
- American College of Obstetricians and Gynecologists. (2017). ACOG Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstetrics & Gynecology, 130(2), e57-e70.
- Harrod, K.S., Hanson, L., VandeVusse, L., et al. (2003). Rh negative status and isoimmunization update: A case-based approach to care. Journal of Perinatal & Neonatal Nursing, 17(3), 166-180.
- Fung, K.F.K., Wong, K., Walsh, J., et al. (2024). Guideline No. 448: Prevention of Rh D alloimmunization. Journal of Obstetrics and Gynaecology Canada. https://www.sciencedirect.com/science/article/pii/S1701216324002603
- de Haas, M., Thurik, F.F., Koelewijn, J.M., et al. (2015). Haemolytic disease of the fetus and newborn. Vox Sanguinis, 109(2), 99-113.
- Cleveland Clinic. (2023). Rhesus (Rh) Factor: Incompatibility, Complications & Pregnancy. https://my.clevelandclinic.org/health/diseases/21053-rh-factor
- RegisteredNurseRN.com. (2018). Rh Incompatibility in Pregnancy NCLEX Review. https://www.registerednursern.com/rh-incompatibility-pregnancy-nclex-review/
- Lecturio Nursing. (2024). Rh Incompatibility [+ Free Cheat Sheet]. https://www.lecturio.com/nursing/free-cheat-sheet/rh-incompatibility-in-pregnancy/
© 2025 Rh Incompatibility Nursing Notes | Created for educational purposes
Focus keyword: Rh incompatibility | Density: ~1.2%
